Fig. 7.1
Activation of a photosensitizer (S) by light (hν) to triplet state (*S) and reaction with oxygen (O2) or other substrate (R) in Type I and II photo processes
The Type I pathway involves electron transfer reactions from the PS triplet state with the participation of a substrate to produce radical ions (free radicals) that can then react with oxygen to produce cytotoxic species, such as superoxide, hydroxyl and lipid-derived radicals.
The Type II pathway involves energy transfer from the PS triplet state to ground-state molecular oxygen (triplet) to produce excited-state singlet oxygen (1O2), which can oxidize many biological molecules, such as proteins, nucleic acids and lipids, and lead to cytotoxicity [19].
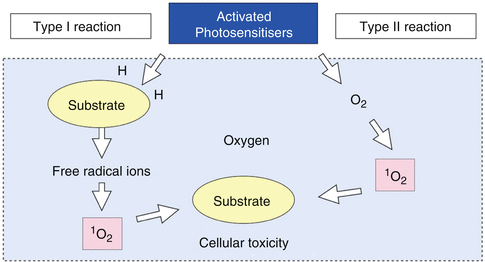

Fig. 7.2
Diagrammatic representation of the different PAD mechanisms (Image courtesy of Giovanni Olivi, Rome, Italy)
The reactive oxygen species (ROS) resulting from both pathways mediate cellular damage. Amongst the ROS, singlet oxygen plays a central role for cytotoxicity in PAD, and the greater the amount of singlet oxygen the target is exposed to, the more effectively cancer cells or bacteria are killed [20, 21].
Many of the target chromophores are cell components that are located close to or are part of the oxidative metabolism (mitochondria) which is essential for the cell vitality. Reactive oxygen species can damage proteins, lipids and other cellular components.
Initially, most studies investigating PAD used photosensitizers developed for use in the treatment of tumours, e.g. the hematoporphyrins, which are reportedly ineffective against gram-negative bacteria [22]. However, Macmillan et al. have shown that other dyes such as toluidine blue can sensitize many bacteria to killing by monochromatic light from a laser [23]. A wide range of PS have been used against pathogenic organisms. They include phenothiazine dyes (methylene blue (MB), toluidine blue O (TBO)), phthalocyanines, chlorins, porphyrins, xanthenes and monoterpene.
Physicochemical parameters such as lipophilicity (log P) and ionization (pKa) are obviously of importance, but other more specialized factors such as light absorption characteristics (the maximum wavelength of absorption λmax, the intensity of the absorption εmax) and the efficiency of singlet oxygen production ΦΔ must be included in a putative photoantimicrobial profile.
Toluidine blue, methylene blue, chlorin e6 conjugates, porphyrin and its derivatives are commonly used photosensitizers in PAD. These photosensitizers can be excited by light sources that fall in the range of red-visible spectrum (usually from 635 to 675 nm) [24].
Indocyanine green is another photosensitizer, activated by wavelengths in the near-infrared spectrum, with a high absorption around 800 nm [25].
Riboflavin and pheophorbide-a polylysine (pheophorbide-a-PLL) are photoactivated by light sources that fall in the range of the visible blue light (380–500 nm) [26].
As of November 2014, few studies have been published on the use of these photosensitizers and laser sources in endodontics (MEDLINE®/PubMed® Resources Guide).
Fundamental to the PAD procedure is the match between photosensitizer and light source. The light source has to emit in the area of the electromagnetic spectrum that coincides with the absorption maximum of the photosensitizer, in order to lift the sensitizer to its triplet state.
In addition, in order to reach the photosensitizer in situ, the penetration depth of the light is also important. Therefore, the wavelengths have to fall within the so called “therapeutic window” of the electromagnetic spectrum between 635 and 1000 nm, the area with the maximum penetration of light into the tissues [24].
Penetration depth in biological tissue for the wavelengths from 635 to 675 nm is about 3–3.5 mm. Wavelengths in the near-infrared spectrum (from 800 to 1000 nm) can penetrate biological tissue deeper, up to 4–5 mm [25].
PAD has also been described as a new therapeutic approach in the management of oral biofilms, with the ability to disrupt the biofilm integrity and affect the biofilm homeostasis [10].
The fact that the active substances are preferentially targeted to the external cell membranes or, when inside, on other intracellular structures virtually eliminates the risk of development of resistance to PAD or inducing gene mutation on the target or surrounding cells. The safety of the procedure makes PAD a treatment strategy which is minimally toxic. The desired/undesired side effect ratio of this treatment is usually high when compared to traditional antimicrobials [27].
High-power lasers, such as the Nd:YAG, have also been reported to be useful for destroying microorganisms, presumably by a thermal effect. Thermal side effect and damage are of concern. PAD in contrast uses low-power light: microbial killing is attained with milliwatts rather than tens or hundreds of watts, without the risk of thermal damage to surrounding structures.
Regarding the effectiveness of the PAD technique, several factors must be considered:
(a)
The fundamental condition for the PAD microbial killing to take place is contact between the photosensitizer and the microorganisms, since the toxic photosensitizer reaction products are short lived and do not act over a great distance. The extent of disinfection thus depends on the extent to which the chemical spreads into the root canal system and dentinal tubules. In this respect, there are no indications that the photosensitizer solution has superior spreading properties than, for example, those of sodium hypochlorite. The advantageous properties of photodynamic disinfection are then limited.
(b)
Type I and II reactions require sufficient oxygenation of the target area. This might not be the case in the anaerobic environment found in primary root canal infections.
(c)
Absorption of energy by the dental tissues could result in an insufficient energy density at the target site, thereby reducing the disinfecting action.
(d)
The wavelengths used must sufficiently penetrate the dental tissues and have adequate differential affinity from the surrounding tissue to avoid energy loss and possible side effects.
(e)
Protocols to remove photosensitizing dyes should be applied after PAD in order to minimize tooth discoloration.
7.3 PAD in Endodontics
In vitro studies on PAD have demonstrated its ability to kill photosensitized oral bacteria such as E. faecalis. More recent in vivo studies demonstrated the microbicidal effect of PAD in the root canal system and also its potential to eradicate persistent endodontic infections for which conventional methods have been unsuccessful [28].
Many studies have investigated the role of photoactivated disinfection as an adjunct to conventional irrigation for root canal disinfection.
Bonsor et al. (2006) published two clinical studies on PAD. The first aimed to assess the efficacy of PAD as an adjunct therapy to conventional root canal disinfection. This in vivo study was performed on teeth with symptoms of irreversible pulpitis or periradicular periodontitis. Microbiological samples were taken from the canals after accessing the canal, after conventional disinfection procedures and after the PAD process. Samples were plated onto anaerobic culture media and incubated for 5 days. Sixteen out of twenty infected canals were negative to culture after conventional endodontic therapy. Three of the four which had remained infected cultured negative after the PAD process. The last sample treated with a fibre was positive for bacteria, but upon inspection the fibre was broken reducing the effective light output by 90 %. The authors concluded that PAD offers potential to eliminate bacteria from the root canals especially where conventional techniques have failed to do so [29].
In a second study, performed in vivo on 64 teeth, Bonsor et al. (2006) compared the use of 20 % citric acid in association with PAD to the use of 20 % citric acid and 2.25 % sodium hypochlorite on the intracanal bacterial load. Samples taken before and after the disinfection therapy were anaerobically cultured. Results indicated that the combination of a chelating agent (citric acid), acting as a cleaner, with the photoactivated disinfection was an effective alternative to the use of sodium hypochlorite for the root canal cleaning and disinfection [30].
Garces et al. (2007) performed an in vitro study on extracted human teeth mechanically prepared and infected with two bioluminescent gram-negative species (Proteus mirabilis and Pseudomonas aeruginosa). The samples were cultured for 3 days to create intracanal biofilms. Conventional endodontic irrigation was compared to PAD using a conjugate between polyethylenimine and chlorin (e6) as photosensitizer activated by a 660-nm diode laser, delivered in the canals through a 200-μm fibre. The conventional therapy alone led to a reduction of bacterial bioluminescence of 90 %, whilst the PDT alone resulted in a reduction of 95 %. The combination of the two therapies resulted in a reduction of more than 98 %. Bacterial regrowth observed 24 h after treatment was much lower for the combined therapy samples compared to the single treatments (P <0.0005) [31].
Foschi et al. (2007) tested aPDT in root canals of extracted teeth experimentally infected with E. faecalis for 3 days. Methylene blue (6.25 μg/mL) was left in the canal for 5 min, then irradiated with 665-nm laser light (60 J/cm2) transmitted through an optical fibre of 500 μm. They observed only a 77.5 % (i.e., <1 log unit) reduction in viable counts and concluded that the photosensitizer concentration and light parameters require optimization to maximize bacterial killing in root canals [32].
Garcez et al. (2008) in another study analysed in vivo the antimicrobial effect of PAD in association with conventional endodontic treatment. They collected microbiological samples after accessing the canal, following conventional instrumentation irrigation and after photoactivated disinfection. Conventional chemomechanical root canal cleaning resulted in a mean reduction of the bacterial load of 1.08 log units. Combination with PDT significantly enhanced the reduction to 1.83 log units. Also a second PDT session in a second visit was significantly more effective than the first. The authors concluded that the use of PDT added to endodontic treatment leads to an increased bacterial reduction [33].
Lim et al. (2009) investigated the efficacy of light-activated disinfection on 4-day-old (immature) and 4-week-old (mature) Enterococcus faecalis biofilms grown within root canals of extracted teeth. Different treatment protocols were investigated: chemical (NaOCl) disinfection, conventional PAD, improved PAD (photosensitizer in a specific solvent system followed by illumination in the presence of an irradiation medium) and chemomechanical disinfection (alone and in combination with improved PAD). In the 4-day-old group, inactivation of bacteria from deeper dentine was higher using improved PAD than using sodium hypochlorite alone. In 4-week-old biofilms, the combination of chemomechanical disinfection and improved PAD produced superior bacterial killing compared to either chemomechanical disinfection or improved PAD alone, highlighting the potential of improved PAD to kill bacteria within dentinal tubules [34].
Garcez et al. (2010) studied in vivo the antimicrobial effect of PAD in combination with conventional endodontic treatment on patients with necrotic pulps and resistant antibiotic microflora. They selected 30 anterior teeth with apical lesions from 21 patients that were previously treated with conventional endodontic treatment and antibiotic therapy. Microbiological samples were taken after accessing the root canal, after conventional endodontic therapy and finally after PAD. Polyethylenimine-chlorin was used as photosensitizer, and a diode laser was used as a light source (40 mW for 4 min, 9.6 J). Conventional endodontic therapy alone produced a significant reduction in numbers of microbial species but only three teeth were free of bacteria. The combination of PAD and conventional endodontic treatment significantly increased the number of bacteria-free teeth and eliminated all drug-resistant species [35].
Rios et al. (2011) evaluated the antimicrobial effect of PAD using toluidine blue O (TBO) in combination with a LED light source after a conventional disinfection protocol with 6 % NaOCl on extracted teeth infected for 2 weeks with Enterococcus faecalis. The bacterial survival rate of the NaOCl/TBO/light group (0.1 %) was found to be significantly lower (P < .005) than the NaOCl (0.66 %) and TBO/light groups (2.9 %), suggesting the potential use of PAD as an adjunctive antimicrobial procedure in conventional endodontic therapy [36].
Poggio et al. (2011) compared the antimicrobial effect of photoactivated disinfection, conventional 5.25 % NaOCl irrigation and a combination of both on Enterococcus faecalis-, Streptococcus mutans– and Streptococcus sanguis-infected teeth. They reported that PAD applied for a longer time or PAD associated with 5 % NaOCl showed significantly higher antibacterial effects [37].
Silva et al. (2012) studied in vivo the response of periapical tissues of dog’s teeth with apical periodontitis after one-session endodontic treatment with and without antimicrobial photodynamic therapy (PAD). Phenothiazine chloride at 10 mg/mL was inoculated for 3 min as a photosensitizer and then photoactivated by a diode laser (660 nm, 60 mW/cm2) for 1 min. After 90 days, sections from the maxillas and mandibles were histologically investigated. Qualitatively and quantitatively, the PAD-treated groups showed periapical region moderately/severely enlarged, with no inflammatory cells, moderate neoangiogenesis and fibrogenesis and the smallest periapical lesions suggesting PAD as a promising adjunct therapy to cleaning and shaping procedures in teeth with apical periodontitis undergoing one-session endodontic treatment [38].
The studies cited so far describe the use of PAD in combination with traditional chemomechanical root canal cleaning. The photoactivated disinfection has also been studied as an alternative method for root canal disinfection.
Fonseca et al. (2008) studied the effects of PAD on root canals of extracted teeth infected with Enterococcus faecalis and incubated for 48 h. A solution of 0.0125 % toluidine blue was used for 5 min followed by irradiation using a diode laser (Ga-Al-As, 660 nm) at 50 mW. The mean decrease in CFU was 99.9 % in the test group, whereas in the untreated control group, an increase of 2.6 % was observed [39].
Fimple et al. (2008) investigated the effects PAD on root canal systems incubated with a combination of Actinomyces israelii, Fusobacterium nucleatum subspecies nucleatum, Porphyromonas gingivalis and Prevotella intermedia. Scanning electron microscopy analysis showed the presence of biofilms in the root canals before therapy. Methylene blue (25 μg/mL) was inoculated into the canals and left for 10 min followed by exposure to red light at 665 nm with a fluence of 30 J/cm2 delivered with a 250-μm fibre. The PAD achieved up to 80 % reduction of colony-forming unit counts [40].
An in vitro apical lesion model was used by Nagayoshi et al. (2011) to study the direct effects of irradiation with a diode laser as well as heat produced by irradiation on the viability of microorganisms in periapical lesions. The combination of a photosensitizer and laser irradiation significantly reduced the viability of E. faecalis. The study also demonstrated that the rise in temperature caused by irradiation was not responsible of the cytotoxic effects on E. faecalis [41].
Stojicic et al. (2013) compared the efficacy of conventional and modified photoactivated disinfection (PAD) against Enterococcus faecalis and mixed plaque bacteria in suspension and biofilms. Conventional PAD, used for 3 min, killed from 90.76 to 100 % E. faecalis but failed to kill all plaque bacteria even after 5 min of laser irradiation. In modified PAD, up to 100 % of suspended E. faecalis and mixed plaque bacteria were killed after 1 min and 30 s of irradiation. Up to twenty times, more biofilm bacteria were killed by modified PAD than by conventional PAD with 15 μmolL MB and up to eight times more than 2 % CHX and 1 % sodium hypochlorite [42].
Bago et al. (2013) evaluated the antimicrobial effect of a diode laser irradiation (2 W, 3 × 20 s), photoactivated disinfection (PAD) (100 mW, 60 s), conventional syringe irrigation (30 gauge with 2.5 % NaOCl, 60s) and sonic activated irrigation (EndoActivator system with 2.5 % NaOCl, 60 s) on Enterococcus faecalis. The PAD and EndoActivator system were more successful in reducing the root canal infection than the diode laser and NaOCl syringe irrigation alone [43].
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


