Resident microbiota
Consists of the common microflora (microorganisms found in most humans), the supplemental microflora (microorganisms characterising the individual) and the transient microflora (microorganisms temporarily present in the body)
Probiotic
Living microorganisms which, when ingested, provide a health benefit on the host
Prebiotic
Non-digestible food ingredients, e.g. oligosaccharides, inulin and lactulose, that stimulate the growth and/or activity of selected beneficial bacteria
Synbiotic
Nutritional supplements combining probiotics and prebiotics synergistically, which beneficially affect the host
During the last decade, the interest in probiotics as an alternative, preventive, and therapeutic approach in the oral cavity has arisen. The efficacy of probiotic bacteria in the oral cavity has been investigated for conditions including dental caries, gingivitis, periodontitis, halitosis, colonisation of oral Candida, oral mucositis and xerostomia. Within dentistry, L. rhamnosus GG and L. reuteri are the most intensely studied probiotic species, and they have shown their potential in interacting with S. mutans and reducing colonisation of Candida. The intention of this chapter is to briefly outline current knowledge on the potential for use of probiotic bacteria to prevent oral diseases and to improve oral and dental health.
12.2 Mechanisms of Action
The mechanisms of action of probiotics are not fully understood but are thought to be locally in the mouth by competing for adhesion sites and nutrients with the oral pathogens and by inhibition of growth of pathogens by production of bacteriocins or other products (acid or peroxide). Thus, probiotics modify the composition of the oral biofilm or the metabolic activity. There also seems to be a systemic regulation of the immune response during intake of probiotics (Devine and Marsh 2009; Stamatova and Meurman 2009). Alterations in levels of both salivary IgA (Ericson et al. 2013) and cytokines in the gingival crevicular fluid (Twetman et al. 2009) have been registered after exposure to probiotic bacteria. Generally, the effects of probiotic bacteria are strain specific and cannot be applied directly to other strains. Also, the same strains can have different effect in different individuals (Koll-Klais et al. 2005).
12.3 Probiotics and Oral Diseases
12.3.1 Dental Caries
Dental caries is demineralisation of the tooth induced by microbial production of acid in the dental plaque. Hence, modulation of the dental plaque by probiotic bacteria has naturally been of interest.
Since the first study on the topic by Näse et al. in 2001, there have been an increasing number of clinical trials with caries-related end points. However, the majority of these studies have other end points than caries such as microbial counts or plaque index. Mutans streptococci are the most common microbial end point, but some studies also look at lactobacilli. Most of these studies show an inhibitory effect of probiotics on salivary mutans streptococci, but a few does not find any difference after the intervention period (Stecksen-Blicks et al. 2009; Lexner et al. 2010; Taipale et al. 2012). Nonetheless, two recent systematic reviews conclude that probiotic bacteria decrease the mutans streptococci counts as long as there is a regular intake of the probiotic bacteria (Cagetti et al. 2013; Laleman et al. 2014). The strains used in the clinical intervention trials vary among different lactobacilli strains and a few different bifidobacteria and even some streptococci strains. There does not seem to be any clear-cut difference between the outcomes of the trials based on the strains used in the study.
Since the lactobacilli used in most studies have strong acidogenic abilities, a natural reservation in relation to caries would be that addition of lactobacilli to the oral cavity would involve an increased risk of an acidogenic shift of the oral microbiota. However, two clinical trials found no increase in biofilm acidurity after exposure to probiotic lactobacilli strains (L. reuteri SD2112, L. reuteri DSM 17938 and L. reuteri PTA 5289) (Keller and Twetman 2012; Marttinen et al. 2012).
There are fewer studies with caries incidence, root caries arrest or remineralisation of carious lesions as outcome. The six studies with caries incidence as end points are listed in Fig. 12.1, which displays the prevented fraction of the studies. The calculated mean of preventive fraction is 33 %. The most popular vehicle for the probiotic bacteria was milk which was supplemented with 2.5 ppm fluoride in one study. The first study from Finland (Näse et al. 2001) also used milk as the vehicle for L. rhamnosus GG during a 7-month intervention period. They found a preventive fraction of 56 % among the children being 3–4 years of age but only 21 % when looking at the entire study population of 1–6 years of age. Besides the dental health benefits, the study also showed a reduction in antibiotic treatments for respiratory infections in the test group (Hatakka et al. 2001). The promising results encouraged a similar study in Sweden where preschoolers aged 1–5 years were provided with milk containing L. rhamnosus LB21 and 2.5 ppm fluoride on a daily basis (Stecksen-Blicks et al. 2009). Their results showed a 75 % preventive fraction but due to the study design, it was not possible to determine how much of this can be attributed to the fluoridation or the addition of probiotic bacteria. Secondly, this study also found an additional beneficial effect in fewer prescriptions of antibiotics to the children in the test group. A more recent study from Finland chose another vehicle based on the young age of the participants (0–12 months) and used a pacifier with room for probiotic tablets to obtain longer oral exposure to the probiotic strain L. rhamnosus LB21 (Taipale et al. 2013). At age four, however, the caries preventive fraction was rather modest (5 %) which might be due to uncertain compliance.
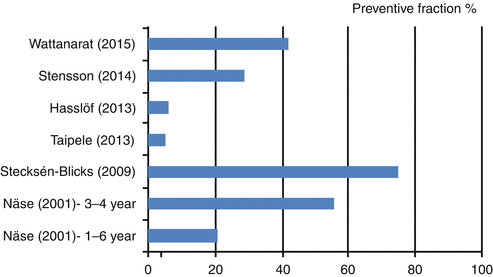

Fig. 12.1
Preventive fractions in clinical studies with caries as an end point
Two Swedish studies are follow-up studies on trials conducted 6 and 7 years ago, respectively. After having eaten gruel with added L. paracasei F19 during infancy, the children in the test group had statistically significant less eczema at age 6 but not statistical significant results on caries incidence (Hasslof et al. 2013). The dropout rate was rather high and together with generally low level of caries, it may account for failure to achieve statistically significant results. In comparison, the results of the other study were more promising. Stensson et al. (2014) reports a preventive fraction of 29 % 7 years after the original intervention with five drops of L. reuteri ATCC a day during the first 12 months of age.
It has been discussed whether colonisation of the probiotic strains is necessary to gain an effect. The studies with microbial end points show a change in levels of mutans streptococci as long as the probiotic strains are administered but mutans streptococci return to previous levels when the probiotic bacteria are ceased. Hence, the effect seems to be dependent of a continuous distribution of the probiotic bacteria. However, the follow-up study by Stensson reported on an effect 7 years after the original intervention. This could point towards the importance of intervention in early childhood to secure a long-term effect. Despite the promising results from the existing studies, there is still insufficient evidence to give any clinical recommendations, and more long-term studies with caries as an end point are needed.
12.4 Gingivitis and Periodontitis
Gingivitis and periodontitis are diseases related to the gum and surrounding bone caused by bacterial dental plaque and the host immune response. Exposure of the gingival tissues to dental plaque results in inflammation within the tissues which manifests in clinical signs of gingivitis, e.g. change in colour, swelling of the tissue, increased gingival exudate and bleeding upon provocation. The clinical features of chronic periodontitis are, in addition to the characteristics of gingivitis, loss of clinical attachment and loss of alveolar bone. Today, treatment strategies include hygiene improvement, mechanical debridement (scaling and root planing), surgery and antibiotic treatment. One of the main etiological factors in periodontal inflammation is the shift of the periodontal microbiota towards gram-negative species and the absence of so-called beneficial bacteria. Theoretically, restoring the reduced number of these beneficial bacteria via probiotic administration might be of interest in the treatment of periodontal disease (Teughels et al. 2008).
A Medline search in June 2015 revealed 24 in vivo human clinical studies concerning the effect of probiotic bacteria on periodontally healthy individuals or patients with gingivitis or periodontitis. Selected studies are shown in Table 12.2. These studies mostly report end points related to amount of plaque (PI), gingival condition (GI), bleeding on probing (BOP), probing pocket depth (PPD) and subgingival microbiota associated with periodontal diseases. In the majority of the studies, a significant effect of probiotic treatment was obtained in the probiotic group compared to placebo. However, the studies are heterogeneous and have been subject to methodological criticism mainly due to a diverse patient population, lack of descriptions of the extent and severity of the periodontal disease, potential confounding factors, high risk of bias and inconsistent end points (Dhingra 2012; Laleman and Teughels 2015).
Table 12.2
Human clinical studies with outcome related to periodontal disease
|
Infection type at baseline
|
Reference
|
Strain
|
Vehicle, time
|
Assessment criteria
|
Effect of probiotic treatment
|
|---|---|---|---|---|---|
|
Healthy volunteers
|
Kang et al. (2006)
|
W. cibaria CSM1
|
Rinse, 1 day
|
OHI-S, PI
|
Yes
|
|
Shimauchi et al. (2008)
|
L. salivarius WB21
|
Tablets, 8 weeks
|
PI, GI, BOP
|
Yes
|
|
|
Iwamoto et al. (2010)
|
L. salivarius WB21
|
Tablet, 4 weeks
|
BOP, PPD
|
Yes
|
|
|
Sinkiewicz et al. (2010)
|
L. reuteri ATCC 55730/ATCC PTA 5289
|
Diet, 12 weeks
|
PI, periopath.
|
Yes
|
|
|
Gingivitis
|
Krasse et al. (2006)
|
L. reuteri
|
Chewing gum, 2 weeks
|
GI, PI
|
Yes
|
|
Twetman et al. (2009)
|
L. reuteri ATCC 55730/ATCC PTA 5289
|
Chewing gum, 2 weeks
|
BOP, GCF, cytokines
|
Yes
|
|
|
Exp.
|
Staab et al. (2009)
|
L. casei Shirota
|
Milk drink, 8 weeks
|
PI, GI, GCF
|
Yes
|
|
Exp.
|
Slawik et al. (2011)
|
L. casei Shirota
|
Milk drink, 4 weeks
|
PI, BOP
|
Yes
|
|
Iniesta et al. (2012)
|
L. reuteri ATCC 55730/ATCC PTA 5289
|
Tablets, 8 weeks
|
PI, GI
|
No
|
|
|
Exp.
|
Hallström et al. (2013)
|
L. reuteri ATCC 55730/ATCC PTA 5289
|
Tablets, 3 weeks
|
PI, GI, BOP
|
No
|
|
Periodontitis
|
Riccia et al. (2007)
|
L. brevis
|
Lozenges, 4 days
|
GI, PI, BOP, calculus
|
Yes
|
|
Tsubura et al. (2009)
|
B. subtilis E-300
|
Rinse, 30 days
|
PPD, BOP, GI
|
No
|
|
|
SRP
|
Vivekanada et al. (2010)
|
L. reuteri DSM 17938/ATCC PTA 5289
|
Lozenges, 3 weeks
|
PI, GI, BOP, PPD, CAL
|
Yes
|
|
Vicario et al. (2013)
|
L. reuteri ATCC 55730/ATCC PTA 5289
|
Tablets, 4 weeks
|
PI, BOP, PPD
|
Yes
|
|
|
SRP
|
Teughels et al. (2013)
|
L. reuteri DSM 17938/ATCC PTA 5289
|
Tablets, 12 weeks
|
PPD, CAL, BOP, periopath.
|
Yes
|
|
SRP
|
Shah et al. (2013)
|
L. brevis
|
Lozenges, 2 weeks
|
GI, PI, PPD, CAL
|
Yes
|
|
Szkaradkiewicz et al. (2014)
|
L. reuteri ATCC PTA 5289
|
Tablets, 2 weeks
|
GI, PPD, CAL
|
Yes
|
|
|
Tekce et al. (2015)
|
L. reuteri DSM 17938/ATCC PTA 5289
|
Lozenges, 3 weeks
|
PI, GI, BOP, PPD
|
Yes
|
Eight clinical studies have looked at clinical periodontal parameters in periodontally healthy individuals (Burton et al. 2013; Iwamoto et al. 2010; Kang et al. 2006; Karuppaiah et al. 2013; Mayanagi et al. 2009; Shimauchi et al. 2008; Sinkiewicz et al. 2010; Zahradnik et al. 2009). In a randomised, double-blind, placebo-controlled trial, Shimauchi et al. (2008) investigated the effect of L. salivarius WB21 on 66 healthy subjects and found periodontal parameters improved after 8-week intervention. Mayanagi et al. (2009) and Zahradnik et al. (2009) found a reduction in selected periopathogens (e.g. P. gingivalis) in subgingival plaque and saliva of healthy individuals after treatment with lactobacilli spp. Two randomised clinical trials have looked into the efficacy of probiotics on gingival health in children. In the first study by Burton and co-workers (2013), 100 children (5–10 years) were included to assess changes in plaque score and gingival score after 3 months treatment with either S. salivarius M18 or placebo. At treatment end, the plaque scores were significantly lower in the probiotic group, but no differences were seen in gingival scores. These findings were confirmed by Karuppaiah et al. (2013).
Krasse et al. (2006) were the first to investigate the effect of chewing gum containing L. reuteri on patients with chronic gingivitis. They found a reduction in PI and GI after 2-week intervention. However, Iniesta and co-workers (2012) were not able to confirm these findings. Three clinical trials have looked upon the effect of probiotics on subjects with experimental gingivitis (Hallström et al. 2013; Slawik et al. 2011; Staab et al. 2009): two with positive and one with a negative outcome (Table 12.2).
Bleeding on probing (BOP) is a widely used criterion to diagnose gingival inflammation. Seven studies found decreased BOP after treatment with probiotic bacteria compared with placebo (Fig. 12.2) (Ince et al. 2015; Slawik et al. 2011; Teughels et al. 2013; Tsubura et al. 2009; Twetman et al. 2009; Vicario et al. 2013; Vivekananda et al. 2010). However, Teughels et al. (2013) did not find this decrease significant.
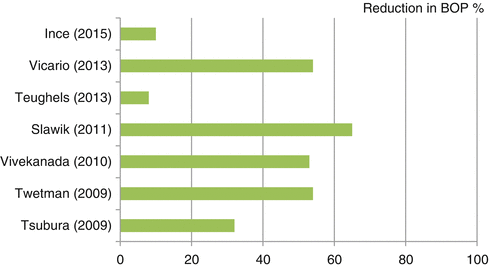

Fig. 12.2
Percent reduction in BOP between probiotic and placebo groups
Nine recent studies (2007–2015) have investigated the effect of probiotics on patients with chronic periodontitis (Ince et al. 2015; Riccia et al. 2007; Shah et al. 2013; Szkaradkiewicz et al. 2014; Tekce et al. 2015; Teughels et al. 2013; Tsubura et al. 2009; Vicario et al. 2013; Vivekananda et al. 2010) (Table 12.2). Longitudinal studies have shown the efficacy of the standard treatment approach consisting of systematic scaling and root planing (SRP) on root surfaces and optimal oral hygiene. Three studies combined the SRP with probiotic treatment. In a randomised placebo-controlled trial from 2013, Teughels et al. proved the effect of L. reuteri (Prodentis)-containing probiotic lozenges as an adjunct to SRP in patients with chronic periodontitis. Significantly larger PPD reductions, especially in moderate and deep pockets, were evident.
Antibiotic treatment can be an adjunct to mechanical therapy when these are found to be insufficient. However, repeated use of antibiotics increases the risk of drug-resistant microorganisms. Shah et al. (2013) compared the efficacy of probiotic tablets (L. brevis CD2) alone, in combination with doxycycline and doxycycline alone after SRP in patients with aggressive periodontitis. The study showed that all three alternatives had similar reducing effect on PI, GI and PPD after 2 months. This finding is encouraging since a reduction in the use of antibiotics in the dental practice is desirable.
In summary, within the limitations of the clinical studies performed to date, the results are encouraging and display that probiotics might be a valid supplement to the gold standard treatment of gingivitis and chronic periodontitis patients. However, further long-term studies with more homogenous end points are needed before evidence-based treatment recommendations can be released.
12.4.1 Colonisation of Oral Candida Species
The yeast Candida can cause a number of disorders in the oral cavity, known as oral candidiasis. Seven Candida species are the clinically most important, of which C. albicans, C. tropicalis and C. glabrata are most frequently isolated (80 %). Candida species are commensal microorganisms in the oral cavity in 40–60 % of the population and only cause disease when disturbances in the oral microbial balance occur (Teughels et al. 2008). Oral candidiasis is therefore often seen in elderly people and is frequently associated with antibiotic treatment, hyposalivation, impaired local or systemic immune system, neglected oral hygiene, dentures and smoking (Anil et al. 2014; Pires et al. 2002; Shay et al. 1997; Torres et al. 2002). A number of antifungal agents are available for the treatment of oral candidiasis, e.g. the polyenes (nystatin) and the azoles (fluconazole). However, since oral candidiasis is caused by an ecological imbalance (dysbiosis) in the oral biofilm that favours fungal overgrowth, a certain interest has been addressed to a bioecological approach for prevention and management.
In the past decade, several clinical studies have investigated the ability of probiotic bacteria to hamper the growth of Candida in the oral cavity. Hatakka and co-workers (2007) were the first to conduct a randomised double-blind, placebo-controlled trial on the effect of probiotics on the prevalence of oral Candida
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


