
12

Treatment of Salivary Lymphoma and Advanced Nonlymphoid Malignant Salivary Gland Neoplasms
The majority of lymphomas involving head and neck sites are secondary “nodal” lymphomas, which most commonly involve cervical lymph nodes as a manifestation of systemic disease. Approximately 10% of all patients with non-Hodgkin’s lymphoma have primary “extranodal” disease originating in the head and neck. The most common sites of primary lymphoma arising in the head and neck include Waldeyer’s ring, the salivary glands, the nasal cavity, the paranasal sinuses, the thyroid gland, and the orbit. More than half of these head and neck lymphomas occur in Waldeyer’s ring, and approximately one third occur in the paranasal sinuses, the nasal cavity, the oral cavity, and the orbit. The remaining 5 to 10% have their origins in the salivary glands. Salivary gland lymphomas are rare, representing only 1.7 to 5% of all neoplastic disorders of the salivary glands, and ∼4.7% of lymphomas from all body sites.1–5
The parotid gland is the most common major salivary gland to be involved with malignant lymphoma. This is not unexpected due to the large number of lymph nodes situated within and near the parotid gland, as well as the presence of lymphoid aggregates within the gland itself. Although the overall incidence of primary parotid lymphoma is only 1 to 4%, this entity represents anywhere from 64 to 93% of all cases of primary salivary gland lymphoma.3,6 A report from the British Salivary Tumor Panel in 1986 estimated that 61% of salivary gland lymphomas arise in the parotid gland, 18% in the submandibular gland, and the remaining 21% in minor salivary glands.7 In a retrospective study of 26 patients with salivary gland lymphoma, 76.9% of cases involved the parotid gland, and 23.1% of cases involved the submandibular gland.1
Patients with primary salivary gland lymphoma usually present with an enlarging painless mass within the salivary gland. Facial nerve involvement is rare.5,6 Whereas non-Hodgkin’s lymphomas occurring in the head and neck region are most common between the ages of 50 and 60 years, the mean age of diagnosis for patients with primary salivary gland lymphomas is ∼65.5 years.1,5 The male to female ratio is 1.6:1 for all extranodal head and neck lymphomas. In contrast, lymphomas arising in the salivary glands, as well as in the orbit and thyroid gland, occur equally or more frequently in women.5 In a retrospective study of patients with salivary gland lymphomas, the female to male ratio was 3.3:1.1
Salivary gland lymphomas are staged as either extra-nodal or nodal.8 Nodal, or secondary, salivary gland lymphoma is an occasional manifestation of systemic non-Hodgkin’s lymphoma, most commonly follicular histology. Extranodal, or primary, salivary gland lymphoma arises from lymphocytes within the salivary gland parenchyma itself. The most common extranodal histology is the marginal zone mucosa-associated lymphoid tissue (MALT) lymphoma. Because all of the salivary glands, with the exception of the parotid gland, are normally devoid of lymphoid tissue, lymphomas arising within the parenchyma of the salivary gland itself are usually derived from infiltration of reactive lymphocytes into the gland, which is seen in certain autoimmune diseases such as Sjoögren’s syndrome. Extranodal primary lymphomas arising within salivary glands are thus fairly rare.6 To satisfy the criteria defining primary extranodal salivary gland lymphoma, the first clinical manifestation of lymphoma must involve a salivary gland, and there must be histological proof that the lymphoma involves the salivary gland parenchyma. In addition, the lymphoid infiltrate must be a monoclonal malignant process, rather than a polyclonal reactive process. Until 1975, only 64 cases of primary salivary gland lymphoma were reported in the literature.9
Defining primary extranodal lymphoma arising in the parotid gland is more difficult than doing so for the other salivary glands (Table 12-1). Small lymph nodes are embedded within the parotid gland but not in the other salivary glands. These intraparotid nodes are complex. They can contain salivary ducts and acini, a phenomenon that reflects the close relationship between the salivary gland and this lymphoid tissue during embryonic development. Given this intimate relationship, it is often difficult for the pathologist to discriminate between primary and secondary parotid lymphoma. Numerous conflicting criteria have been described in the literature to define primary parotid gland lymphoma. Some authorities indicate that any lymphoma originating in the parotid gland represents a primary parotid lymphoma, even if the lymphoma originates from intraglandular lymph nodes, as long as the parotid tumor is the only clinical manifestation of lymphoma. Other authors strictly define a parotid lymphoma as a lymphoma that must originate in glandular parenchyma.8 Most experts use this latter definition and reserve the diagnosis of primary parotid salivary gland lymphoma for a lesion that contains no discernible lymph nodes or visible lymph node capsule surrounding the lymphoid infiltrate.10
 Pathogenesis and Risk Factors
Pathogenesis and Risk Factors
Sjoögren’s Syndrome
A reactive salivary gland inflammatory process known as myoepithelial (lymphoepithelial) sialadenitis (MESA or LESA) occurs in patients with Sjoögren’s syndrome. MESA has also been called Mikulicz’s disease.4,11 MESA is a benign lymphoid infiltration of the salivary gland resulting in destruction and atrophy of the acinar ducts, in conjunction with proliferation of basal epithelial cells that form “epimyoepithelial islands.” The lymphocytes of MESA are reactive B cells that have infiltrated into the organ, due to autoimmune stimulation. MESA is found in virtually all patients with Sjoögren’s syndrome (see Figs. 5–11, 5–12).5,11
Table 12-1 Criteria for Extranodal or Primary Salivary Gland Lymphoma
| The first clinical manifestation of lymphoma must involve a salivary gland. |
| It must arise from lymphocytes within the salivary gland parenchyma itself. |
| There must be histological proof that the lymphoma involves the salivary gland parenchyma. |
| The lymphoid infiltrate must be a monoclonal malignant process. |
| It contains no discernible lymph nodes or visible lymph node capsule surrounding the lymphoid infiltrate. |
The lymphoepithelial lesion of MESA may undergo clonal expansion in a multistep process, resulting in evolution to lymphoma in ∼4 to 7% of patients with Sjoögren’s syndrome12 (Table 12-2). A chronic inflammatory stimulus represents the initial event, and subsequent sequential proto-oncogene activation may eventually lead to lymphoma. An evolution of histopathologic findings begins with early myoepithelial sialadenitis, which is characterized by focal and organized lymphoid infiltration into Peyer’s patchlike structures surrounding dilated epithelial ducts. (Mucosa-associated lymphoid tissue is normally found in intestinal mucosa, and in this location the lymphoid aggregates are known as Peyer’s patches.) In established MESA, the polyclonal lymphoid infiltrate becomes dense and confluent, and the epimyoepithelial islands proliferate. Transformation of established MESA into lymphoma occurs when the lymphoid infiltrate around the epimyoepithelial islands becomes monoclonal. In frank lymphoma, monomorphic monoclonal lymphocytes that demonstrate immunoglobulin light chain restriction infiltrate the entire salivary gland.3,13
Molecular studies have shown that in both MESA lymphoid cells and MALT lymphoma monoclonal B cells, a B-cell receptor derived from a restricted subset of both B-region and D-region immunoglobulin genes is expressed, consistent with B-cell stimulation by a common or restricted antigen group. This finding supports the hypothesis that chronic stimulation by autoantibodies, which are presumably produced by exposure to exogenous antigens with similarities to self-antigens, plays a role in the lymphoproliferative process by selecting out a specific B-cell subset. B-cell clones are detected in over 50% of patients with MESA when molecular genetic methods are utilized. Finding these B-cell clones does not necessarily correlate with immediate transformation to frank lymphoma. In patients with Sjoögren’s syndrome who undergo salivary gland biopsies revealing MESA, ∼25 to 80% of patients also have immunophenotypic evidence of low-grade MALT lymphoma, which was not clinically apparent. Of these patients, between 10 to 45% eventually develop frank salivary gland lymphoma, often after very long time intervals.4,14
Table 12-2 Multistep Clonal Expansion of the Lymphoepithelial Lesion of MESA
| 1. | MESA is a benign lymphoid infiltration of the salivary gland resulting in destruction and atrophy of the acinar ducts, in conjunction with proliferation of basal epithelial cells that form “epimyoepithelial islands.” |
| 2. | The lymphocytes of MESA are reactive B cells that have infiltrated into the organ, due to autoimmune stimulation (i. e., Sjoögren’s syndrome). |
| 3. | A chronic inflammatory stimulus represents the initial event. |
| 4. | Focal and organized lymphoid infiltration into Peyer’s patch-like structures surround dilated epithelial ducts. |
| 5. | The polyclonal lymphoid infiltrate becomes dense and confluent, and epimyoepithelial islands proliferate. |
| 6. | Proto-oncogene activation transforms established MESA into lymphoma when the lymphoid infiltrate around the epimyoepithelial islands becomes monoclonal. |
| 7. | In frank lymphoma, monomorphic monoclonal lymphocytes that demonstrate immunoglobulin light chain restriction infiltrate the entire salivary gland. |
MESA, myoepithelial sialadenitis
Patients with Sjoögren’s syndrome have a 43.8-fold higher incidence of lymphoma compared with age-matched controls, and ∼20% of all salivary gland lymphomas are associated with Sjoögren’s syndrome. The lymphomas that arise in the context of Sjoögren’s syndrome are primarily low-grade B-cell lymphomas, including extranodal marginal zone MALT lymphoma, small lymphocytic lymphoma, and follicular center cell lymphoma. MALT lymphoma accounts for 75 to 80% of lymphomas that develop in patients with Sjoögren’s syndrome. Fifteen to 25% are intermediate-grade or high-grade B-cell lymphomas, which were likely MALT lymphomas that subsequently transformed to high-grade entities.4,6,14
Helicobacter pylori
Helicobacter pylori has been implicated as the main agent responsible for the development of MALT lymphomas in the stomach. Thus it has been postulated that H. pylori might also play a role in the pathogenesis of salivary gland MALT lymphomas.15 Although H. pylori organisms can be demonstrated in the saliva of patients with gastric H. pylori infection 75% of the time, reports of direct infection of salivary gland tissue itself are sparse.16 If H. pylori does play a direct role in the development of some MALT lymphomas, it would seem to be a relatively unusual occurrence. Jordan et al17 analyzed 20 biopsies obtained from patients with MESA. All biopsies had histological features of organized MALT, and 14 cases showed immunoglobulin heavy chain gene monoclonality consistent with MALT lymphoma. None of these biopsies contained H. pylori deoxyribonucleic acid (DNA). These authors concluded that H. pylori does not play a significant role in the pathogenesis of most MALT lymphomas of the salivary gland.17
There have been reports of salivary gland lymphomas regressing after antibiotic therapy appropriate for Helicobacter pylori, even if no H. pylori organisms were identified within the gland.18 Recirculation of organism-associated antigens from another site of H. pylori infection may lead to antibody production with cross-reactivity to the salivary gland, leading to inflammation and subsequent lymphoma. This hypothesis would explain the favorable effect of antibiotics on the malignant salivary gland process, despite the absence of H. pylori directly involving salivary gland tissue.15,18
Epstein-Barr Virus
Epstein-Barr virus (EBV) is a DNA gamma herpes virus that infects epithelial cells of the oropharynx and a subset of B lymphocytes. Epstein-Barr virus infection has been linked to several malignancies, including nasopharyngeal carcinoma, Burkitt’s lymphoma, T-cell non-Hodgkin’s lymphoma, Hodgkin’s disease, and other lymphoproliferative disorders in immunodeficient individuals. Most EBV-associated malignancies occur in the head and neck region.19
It has been postulated that EBV may be implicated in the pathogenesis of salivary gland lymphomas. In a study of 50 patients with primary non-Hodgkin’s lymphomas arising in the salivary gland and oral cavity, none of these lymphomas were Epstein-Barr virus positive. These results would seem to indicate that EBV is not involved in the pathogenesis of oral and salivary gland lymphomas, at least in immunocompetent patients.19 In contrast, a prospective pathologic study of tissue samples from 19 consecutive patients with salivary gland lymphomas identified Epstein-Barr virus DNA in one sample from a patient with parotid gland lymphoma.20 In another study of 45 patients with MESA, EBV DNA was detected in 3 of 36 samples from patients with salivary gland lymphoma, which represents an 8% incidence of EBV infection. This correlation statistic is similar to figures published for other lymphoma types.21 Finally, the incidence of EBV involving salivary gland tissue is higher for T-cell lymphomas, compared with B-cell lymphomas. It has been estimated that ∼50% of T-cell salivary gland lymphomas may be caused by EBV infection of lymphocytes.22
Post-transplantation Lymphoproliferative Disorder
Post-transplantation lymphoproliferative disorders (PTLDs) occur in 2 to 10% of patients who have received prolonged immunosuppression in the context of both solid organ and bone marrow transplantation. The spectrum of PTLDs includes benign disorders such as plasma cell hyperplasia and polymorphic B-cell hyperplasia, and malignant entities such as B-cell lymphoma and multiple myeloma. PTLD B-cell lymphomas are usually low-grade non-Hodgkin’s lymphomas that are associated with Epstein-Barr virus infection.23 Salivary gland lymphomas are rarely described in the post-transplant setting. In one report, two cases of parotid gland MALT lymphoma arising in the post-transplant setting had no evidence of EBV involving lymphoid tissue by in situ hybridization.23
Human Immunodeficiency Virus
Salivary gland enlargement, due to infiltration of reactive lymphocytes, has been described in patients infected with human immunodeficiency virus (HIV). The pathologic characteristics of these salivary gland infiltrates include glandular atrophy, follicular hyperplasia, cystic dilation of ducts with squamous metaplasia, and the presence of epimyoepithelial islands. These pathologic findings have been described by a variety of terms, including benign lymphoepithelial lesion (BLL), lymphoid hyperplasia, and HIV-associated salivary gland disease. HIV-1 major core protein, P-24, as well as HIV-1 ribonucleic acid (RNA) sequences have been found within follicular dendritic cells of salivary glands containing HIV-associated BLL. Epstein-Barr virus DNA has also been reported in salivary gland tissue of some patients with HIV-associated BLL. HIV-associated BLL is considered a risk factor for salivary gland lymphoma.24
In a study of six cases of primary salivary gland lymphoma occurring in patients with HIV infection, one patient had small noncleaved cell Burkitt’s lymphoma, two patients had large cell immunoblastic lymphoma, and the remaining three had large cell intermediate-grade non-Hodgkin’s lymphoma. Epstein-Barr antigens were detected by in situ hybridization for EBV RNA in three of the six HIV-related lymphomas. In all cases, the HIV p24 antigen was selectively deposited in the germinal centers and follicular dendritic cells of salivary gland tissue.25
The salivary gland lymphomas arising in patients with HIV disease are rarely MALT lymphomas. Some cases of MALT lymphoma have been reported in pediatric patients with HIV disease. In one report, four HIV-1 positive pediatric patients were found to have MALT salivary gland lymphoma. The pathogenesis of the MALT lesions that occur in patients who are positive for HIV infection is unclear. The HIV retrovirus may play a direct role. Alternatively, EBV may be involved in the pathogenesis of these lesions.26
Hepatitis C
There is a correlation between hepatitis C virus infection and extranodal MALT lymphomas, including salivary gland MALT lymphomas.27 Hepatitis C virus antigen has been found within the salivary gland epithelial cells from patients with parotid MALT lymphomas. Hepatitis C virus may act as an exogenous antigenic stimulus that leads to B-cell proliferation. Alternatively, the virus may contribute to the development of the multistep process of lymphomagenesis.3
Hepatitis C virus has also been implicated in the pathogenesis of lymphomas occurring in patients with the autoimmune disease known as mixed cryoglobulinemia. Homologies have been demonstrated between the antigen combinatory regions of the antigen receptor variable region genes expressed in both hepatitis C virus–associated non-Hodgkin’s lymphoma tissue in patients with mixed cryoglobulinemia, and non-neoplastic salivary gland MESA arising in the context of Sjoögren’s syndrome. Given the similarities, an immunologic cross-reactivity may exist between hepatitis C virus and the unknown stimulating agent that underlies both of these disorders.28
Kuöttner’s Tumor (Chronic Sclerosing Sialadenitis)
In 1896, Kuöttner described a series of patients with a unilateral hard tumor-like mass involving the submandibular gland. Histologically, this entity showed evidence of chronic sclerosing sialadenitis. Kuöttner’s tumor is not associated with Sjoögren’s syndrome or any other autoimmune disease. The pathogenesis of chronic sclerosing sialadenitis is duct obstruction by abnormal secretions. The salivary gland epithelial ducts are typically dilated and filled with secretions, and sialoliths are found in 50 to 80% of the cases. Periductal fibrosis, lobular fibrosis, and acinar atrophy are seen and associated with a lymphoplasmacytic infiltrate. The presence of marked fibrosis and the lack of epithelial proliferation distinguish sclerosing sialadenitis from MALT.11 MALT lymphoma has been described in association with chronic sclerosing sialadenitis. Several case reports suggest that chronic sclerosing sialadenitis is a risk factor for low-grade MALT salivary gland lymphoma.11
Human Herpes Virus-8
Human herpes virus-8 (HHV-8) may rarely be involved in Sjoögren’s syndrome–associated MALT lymphomas. HHV-8 DNA sequences have been detected in tissue from one patient with a parotid gland MALT lymphoma. This patient was an HHV-8 seropositive woman with Sjoögren’s syndrome. Although HHV-8 may trigger MALT lymphomas in patients with Sjoögren’s syndrome, HHV-8 does not usually infect salivary gland epithelium and probably plays a minimal role in the etiology of epithelial salivary gland neoplasms.29,30
 Pathology
Pathology
Histological Classification
Most salivary gland lymphomas are non-Hodgkin’s lymphomas of the B-cell type. T-cell non-Hodgkin’s lymphoma and Hodgkin’s disease involving the salivary glands are very rare.3 Fifty-five to 70% of salivary lymphomas are low-grade lymphomas, such as extra-nodal marginal zone mucosa-associated lymphoid tissue lymphoma, small lymphocytic lymphoma, and follicular lymphoma. The majority of low-grade salivary gland lymphomas are MALT lymphomas, which are described in more detail below.6 Approximately 31 to 36% of salivary gland lymphomas have an intermediate-or high-grade histology.6 Some intermediate-and high-grade salivary gland lymphomas are originally derived from MALT or follicular lymphomas and become more aggressive lymphomas by the process of transformation.3 Most high-grade lymphomas in the salivary glands are diffuse large B-cell lymphomas. Rare cases of lymphoblastic lymphoma and Burkitt-like lymphoma have been reported.4 Cytological diagnosis is possible with fine-needle aspiration and flow cytometry, but often tissue diagnosis with histology is necessary.
Forty cases of primary salivary gland lymphoma were reported to the British Salivary Gland Tumor Panel between 1971 and 1984. This number represents 1.7% of the total collection of 2340 salivary gland tumors referred to the panel during this time frame. There were two cases of nodular sclerosing Hodgkin’s disease, both arising in the parotid gland. The remaining cases were all non-Hodgkin’s lymphomas, 64% of which were low-grade and 36% intermediate-grade by the working formulation histological classification.7
Mucosa-associated Lymphoid Tissue Lymphomas
Mucosa-associated lymphoid tissue lymphomas were first described by Isaacson and Wright in 1983,7a when they described a small series of patients with low-grade B-cell lymphomas arising in gastrointestinal tract mucosa. Although MALT lymphomas occur most frequently in the stomach, they can also occur in various nongastrointestinal sites that are embryologically derived from the foregut, such as the thyroid, lung, breast, orbit, conjunctiva, and salivary gland. Nongastrointestinal locations represent ∼30 to 40% of all low-grade MALT lymphomas.27,31
Mucosa-associated lymphoid tissue is normally found in intestinal mucosa, known as Peyer’s patches. MALT lymphomas arise in sites that normally contain no mucosa-associated lymphoid tissue, such as the salivary gland and stomach. Because salivary glands do not normally contain mucosa-associated lymphoid tissue, the histological organization of MALT lymphoma is acquired in these organs as a result of chronic antigenic stimulation, either in the context of infection or autoimmune disease. Some examples include Helicobacter pylori colonization in the stomach, bronchiectasis in the lung, myoepithelial sialadenitis in Sjoögren’s syndrome, and Hashimoto’s thyroiditis in the thyroid gland. The histological features of mucosa-associated lymphoid tissue are retained in these organs after lymphoid transformation from reactive tissue to MALT lymphoma.18,24,27,31,32
In the Revised European-American Lymphoma (REAL) classification system for lymphomas, published in 1994, the MALT lymphomas are classified among the marginal zone B-cell lymphomas.27 The marginal zone lymphomas are monoclonal B-cell proliferations, which represent neoplasms derived from lymphocytes that reside in the B-cell-rich marginal zone located external to the mantle zone within lymph nodes (Fig. 12–1). The marginal zone is well defined in the spleen and in intestinal Peyer’s patches, but it is very poorly demarcated in peripheral lymph nodes. Subtype entities of marginal zone lymphoma include the extranodal mucosa-associated lymphoid tissue lymphomas, the nodal monocytoid B-cell lymphomas, and the splenic marginal zone lymphomas.32
The central feature of MALT lymphomas is the lymphoepithelial lesion. Aggregates of neoplastic centrocyte-like cells arise in the marginal zone and subsequently infiltrate glandular epithelium in a destructive fashion.27 Findings from immunohistochemistry for salivary gland lymphomas are similar to immunohistochemistry profiles of non–salivary gland lymphomas. MALT lymphomas may have a similar histological appearance to other low-grade lymphomas, such as B-cell small lymphocytic lymphoma and mantle cell lymphoma, but immunohistochemistry can discriminate MALT lymphomas from the other low-grade B-cell lymphomas. Marginal zone lymphomas including MALT lymphomas are positive for the B-cell antigens CD-19, CD-20, and CD-22. They are also positive for CD79A. In contrast to small lymphocytic lymphoma, they are negative for CD-5, CD-10, and cyclin D-1 (BCL-1).12 Expression of CD-43 and CD-23 is variable. MALT lymphomas arising in salivary glands express monoclonal surface immunoglobulin, including IgM, IgG, and IgA, but not IgD.32,33 The variable (V) regions of the immunoglobulin gene in these lymphomas show evidence of somatic mutation, indicating a post-germinal center memory cell stage of differentiation.4,34
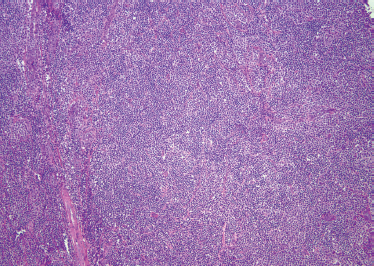
FIGURE 12-1 Mucosa-associated lymphoid tissue (MALT) lymphoma comprised of predominantly small, mature-appearing lymphocytes with scattered monocytoid and plasmacytoid cells in the background (H&E, ×40).
Follicular center cell salivary gland lymphomas are positive for CD-10 and BCL-2.34 Kojima et al10 examined 20 cases of primary salivary gland lymphoma. Of these they identified six patients with follicular lymphoma. Immunohistochemistry showed that all six cases were CD-10 positive, CD-79A positive, BCL-6 positive, CD-3 negative, CD-5 negative, CD-21 negative, CD-23 negative, and cyclin D-1 (BCL-1) negative. The tumor cells expressed BCL-2 in three cases and P-53 onco-protein in four cases.10
T-Cell Lymphoma
Primary T-cell lymphoma of the salivary gland is rare. A recent literature review found only 14 cases of primary T-cell lymphoma of the salivary gland, with all but two of these cases occurring in patients in the East. Parotid and submandibular glands are the most frequently involved, and the most frequent histological type is peripheral T-cell lymphoma of low-grade morphology. T-cell lymphomas of the salivary gland can mimic a low-grade extranodal marginal zone B-cell lymphoma, but they are usually not associated with preexisting sialadenitis or evidence of an underlying autoimmune disease.22 Other T-cell salivary gland lymphomas identified in the literature include angiocentric T-cell/NK-cell lymphoma and T-cell anaplastic large cell lymphoma. Angiocentric lymphoma is a high-grade lymphoma, with a poor prognosis.35
Hodgkin’s Disease
The vast majority of parotid gland lymphomas are of the non-Hodgkin’s type. Hodgkin’s lymphoma involving the salivary glands has been described but is exceedingly rare. Only 32 cases of Hodgkin’s lymphoma arising in the parotid gland have been reported in the English-language literature. Lymphocyte predominant histology seems to be the most common subtype affecting the parotid gland. This is in contrast to the classic presentation of nodal Hodgkin’s disease, which is most commonly the nodular sclerosing histological subtype.36
Plasma Cell Neoplasm
Primary extramedullary plasmacytomas are uncommon tumors that can occur in the head and neck region. These lymphoid malignancies usually involve the submucosal tissue of the upper airway, such as the nasal passages, sinuses, and nasopharynx. Primary extramedullary plasmacytoma involving the salivary glands is rare, and thus usually appears in the literature as single case reports. Amyloid deposition is noted in ∼25% of cases of extramedullary salivary gland plasmacytoma. Treatment consists of surgical resection or more commonly radiation therapy. The prognosis is good.37,38
 Genetics
Genetics
Trisomy 3
The single most common cytogenetic abnormality in salivary gland and extrasalivary gland MALT lymphomas is trisomy 3. In situ hybridization studies with chromosome-specific probes have found trisomy 3 in the interphase nuclei of ∼60 to 70% of cases of low-grade MALT lymphomas. Up to 80% of salivary gland MALT lymphomas contain the trisomy 3 abnormality.24,32
Translocation t(11, 18)
The t(11, 18) translocation is associated exclusively with low-grade extranodal MALT lymphoma. This translocation is observed in up to 50% of extranodal MALT lymphoma cases but it is not observed in other marginal zone lymphomas or in extranodal large B-cell lymphoma. The translocation can be found in tumors of the salivary gland and lacrimal gland, as well as other MALT-associated sites.4,12 Ott et al39 analyzed the cytogenetics of 44 MALT lymphomas arising in the stomach, parotid gland, thyroid gland, lung, breast, and conjunctiva. Fifty-three percent of the low-grade lymphomas displayed the t(11, 18) translocation. In contrast, this same translocation was not found in a single case of high-grade lymphomas arising from MALT.39
The genes involved in the t(11, 18) translocation are not entirely clear. Some candidate genes include the DCC gene, the YES proto-oncogene, the ITF2 gene, the SSAV-related endogenous retroviral element, and the DPC4 gene. Kalla et al40 identified a fusion transcript consisting of the AP12 gene, which is an inhibitor of apoptosis located on chromosome 11, and the MALT-1 gene located on 18 (q-21), in 18 out of 24 gastric and extragastric MALT lymphomas. This fusion protein may interfere with the regulation of apoptosis, which is normally performed by the antiapoptotic function of the AP12 gene product.39,40
Translocation t(14, 18) (BCL-2)
There is usually molecular genetic evidence of the t(14, 18) BCL-2 translocation in follicular center cell low-grade non-Hodgkin’s lymphoma arising in the salivary gland.34 Kerrigan et al41 investigated the presence or absence of the BCL-2 chromosome translocation using molecular techniques in a series of patients with non-Hodgkin’s lymphoma involving the salivary glands. Of the seven cases examined, three had molecular evidence of a t(14, 18) translocation. The four cases lacking BCL-2 rearrangement had diffuse growth patterns. All three cases with BCL-2 rearrangements were nodular lymphomas that arose in patients without Sjoögren’s syndrome or other autoimmune disease. These cases lacked histological evidence of MESA, and thus did not represent MALT-derived lymphomas. These BCL-2-positive salivary gland lymphomas were likely non-MALT-derived follicular lymphomas, which appeared to arise in lymph nodes adjacent to the salivary glands rather than arising in the salivary gland itself.41 In contrast, other authors have described the BCL-2 translocation in MALT lymphomas.24 Pisa et al42 observed the t(14, 18) BCL-2 proto-oncogene translocation in five of seven Sjoögren syndrome–associated MALT lymphomas using Southern blot analysis.
 Treatment for Salivary Gland Lymphoma
Treatment for Salivary Gland Lymphoma
Natural history and prognosis depend not only on the histological subtype of lymphoma but also on the classification of the salivary gland lymphoma as either secondary (nodal) or primary (extranodal). Many patients with secondary nodal salivary gland lymphoma have evidence of systemic disease, whereas patients with primary extranodal salivary gland lymphomas arising from lymphocytes involved in a chronic inflammatory process such as MESA are more likely to have localized disease. A retrospective study showed that patients with primary extranodal salivary gland lymphoma have a favorable prognosis, with more than double the median time to disease progression compared with patients with secondary nodal lymphomas (27.6 months vs 13.3 months). Only 7.7% of patients with primary extranodal lymphomas developed disseminated disease, compared with 46.1% of patients with secondary nodal lymphomas. Lymphoma-related death occurred in 53.8% of the patients with secondary nodal lymphoma, and only 7.7% of patients with primary extranodal lymphoma.1
The median survival for all lymphomas originating within the major salivary glands is ∼49 months.6 Intermediate and high-grade lymphomas have a shorter natural history than do low-grade indolent lymphomas, as expected. Low-grade lymphomas, especially those with MALT histology, tend to remain localized to the primary site for long periods of time. They have a very long natural history and a favorable prognosis.27 Follicular lymphomas arising from the salivary glands appear to have many of the prognostic characteristics of MALT lymphoma arising in the salivary glands, perhaps because they often arise in the context of MESA, as do the MALT lymphomas. They have an indolent natural history and a favorable prognosis.10
Localized treatment approaches, such as resection and/or irradiation, are adequate and often curative for patients with localized primary salivary gland lymphoma. A systemic approach to disease is required in patients with secondary nodal salivary gland lymphoma or in patients with primary extranodal lymphoma with spread to extrasalivary sites.1 The most appropriate treatment approach naturally depends not only on the stage of disease but also on the histological identity of the lymphoma in question.
Treatment for Localized MALT Lymphoma
In a retrospective analysis of 75 patients with nongastrointestinal MALT lymphoma, patients were treated according to both disease stage and site. Patients with localized MALT lymphoma were generally treated with surgery or involved field radiation therapy, and some patients received adjuvant chemotherapy. Most advanced-stage patients received chemotherapy, either consisting of a single agent such as chlorambucil, cyclophosphamide, or fludarabine or a multiagent chemotherapy regimen such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone). Of these 75 patients, only 6 had salivary gland lymphomas. All of the patients with salivary gland lymphoma received chemotherapy, and two of them received involved field radiation therapy as well. Four of the patients (67%) achieved a complete remission, and two of the patients (33%) achieved a partial remission. No firm conclusions can be drawn regarding salivary gland lymphomas from this study because the sample size in this analysis was small.27
Tsang et al43 reviewed the records of 75 patients with biopsy-proven MALT lymphomas arising in extralymphatic organs that were all diagnosed and treated from 1989 to 1998 at Princess Margaret Hospital. Of these patients, 15 (21.4%) had MALT lymphomas arising in a salivary gland, 13 of which were located in the parotid gland. Of the 13 patients with parotid gland lymphoma, 3 patients eventually relapsed after receiving radiotherapy. These MALT lymphomas respond extremely well to moderate-dose-involved field radiation, with dose ranges from 17.5 to 40 Gy.43 Lower doses are used for conjunctival and orbital adnexal salivary gland disease. A median of 33.3 Gy has been reported for use in parotid salivary gland lymphomas.44
A small randomized study of 39 patients with early-stage low-grade marginal zone MALT B-cell lymphomas compared radiotherapy alone to chemo-irradiation for patients with salivary gland MALT lymphomas. Overall, survival and failure-free survival were 90% at 5 years. There was no significant difference between the treatment groups. Thus radiation without chemotherapy would seem most appropriate in this setting.45
Treatment for Advanced-Stage Extranodal MALT Lymphoma
Patients with advanced-stage extranodal MALT lymphomas involving multiple sites of disease should be treated with chemotherapy. Options include cladribine (2-CDA), single-agent oral alkylating agent chemotherapy (cyclophosphamide or chlorambucil), multiagent chemotherapy utilizing CVP (cyclophosphamide, vincristine, and prednisone) or CHOP for six cycles, immunotherapy (anti-CD20 monoclonal antibody therapy utilizing rituximab), or chemoimmunotherapy (R-CHOP).44
Purine Analogues in MALT Lymphoma
The purine analogues are cytotoxic agents that are specific for lymphoid cells, inducing apoptosis in both resting and dividing cells. Because they target cells that are not in the process of dividing, they are promising therapeutic agents for use in indolent lymphomas that have a low proliferative index. The purine analogues 2-CDA and fludarabine have single-agent efficacy in previously treated and untreated indolent non-Hodgkin’s lymphomas. The purine analogues are considered appropriate as first-or second-line therapy for low-grade MALT lymphomas, including those arising in the salivary glands.32
Several studies have demonstrated promising results with the purine analogues in patients with low-grade salivary gland lymphomas. Jager et al46 treated 19 patients with gastric MALT lymphoma and 7 patients with extragastric MALT lymphoma who were chemotherapy naive with 2-CDA. Three patients in this study had MALT lymphoma arising in the parotid gland, and two in the lacrimal gland. Eighty-four percent of patients overall achieved a complete response. One hundred percent of patients with gastric presentation achieved a complete response, and 43% of patients with extragastric presentation achieved a complete response. 2-CDA thus appears to be an active agent in MALT lymphoma involving the salivary glands.46
Treatment for Follicular Lymphoma
Patients with stage III or IV low-grade follicular salivary gland lymphomas should receive chemotherapy or chemoimmunotherapy. Options include CVP, CHOP, oral alkylating agents such as chlorambucil and cyclophosphamide, or anti-CD-20 monoclonal antibody therapy utilizing rituximab.5
Treatment for Diffuse Large Cell Lymphoma
Recommendations for grade I or II intermediate-grade diffuse large cell salivary gland lymphomas include three cycles of CHOP followed by radiation therapy, versus three cycles of R-CHOP followed by involved field radiation therapy. Stage III or IV large cell lymphoma should be treated with standard R-CHOP or CHOP chemotherapy for six to eight cycles and no radiation therapy.5
Treatment for Salivary Gland Plasmacytomas
The treatment of choice for salivary gland plasmacytoma is radiotherapy. Local control is achieved in∼75% of patients. The regional lymphatics should be electively irradiated as well. Even if the salivary gland has been excised, postoperative radiation therapy should be considered. In the absence of radiation therapy, 29% of patients who are status postresection develop lymph node metastases, and 21% of patients develop systemic disease.38
 Treatment for Nonlymphoid Salivary Gland Malignant Tumors
Treatment for Nonlymphoid Salivary Gland Malignant Tumors
Surgery and Radiation for Localized Disease
The majority of salivary gland carcinomas are initially treated with surgical resection. After complete excision, adjuvant radiation is considered for select histological subtypes of salivary gland carcinoma. Patients with small, mobile primary tumors with low-grade histology are managed with surgical resection alone.47–50
Chemotherapy for Recurrent and Advanced Disease
The overall incidence of distant metastases for patients with malignant salivary gland tumors is ∼25%.49 These patients will eventually succumb to their disease. Chemotherapy can often palliate symptoms and in some cases prolong survival. In general, the most active agents in metastatic salivary gland carcinomas include cisplatin, doxorubicin, and 5-fluorouracil (5-FU).47 Other agents with activity include chlorambucil, hydroxyurea, hexamethylmelamine, daunorubicin, cyclophosphamide, mitomycin C, methotrexate, bleomycin, and vincristine.51 Newer agents with promise include the taxanes and vinorelbine.47
The most widely used three-drug chemotherapy combination for patients with advanced salivary gland carcinomas is the CAP (cyclophosphamide, doxorubicin, and cisplatin) regimen. In one study of 22 patients with a variety of tumor histology including adenoid cystic carcinoma, salivary duct carcinoma, adenocarcinoma, mucoepidermoid carcinoma, nonkeratinizing undifferentiated carcinoma, and neuroendocrine small cell cancer, the CAP regimen provided an overall response rate of 27%. CAP thus appears to be a moderately active combination chemotherapy program for all patients with advanced salivary gland cancer, regardless of tumor histology.52
Suen and Johns53 evaluated 85 cases of salivary gland cancers treated with chemotherapy and assessed the most active drugs for each histological class of tumors. The overall response rate to chemotherapy was 42%. Chemosensitivity seemed to depend on tumor histology. The adenocarcinoma-like cancers (adenoid cystic carcinoma, adenocarcinoma, malignant mixed tumor, and acinous cell tumors) had the highest response rates to doxorubicin, cisplatin, and 5-FU. The squamous-like cancers (squamous cell carcinoma and mucoepidermoid carcinoma) responded well to methotrexate and cisplatin.53 Other authors have also commented on this chemosensitivity pattern. Kaplan et al54 reviewed the results of 15 chemotherapy trials containing a total of 116 patients with advanced salivary gland cancers. Adenoid cystic carcinoma, adenocarcinoma, malignant mixed tumor, and acinic cell carcinoma had similar chemotherapy sensitivities. For this “adenocarcinomalike” group of tumors, the most active agents included cisplatin, 5-FU, and doxorubicin, and an enhancing effect was seen between cisplatin and doxorubicin. Cyclophosphamide and methotrexate were ineffective. In this pooled analysis, high-grade mucoepidermoid carcinoma appeared to share a similar chemosensitivity spectrum with squamous cell carcinoma of the head and neck. Both histological types demonstrated a 36% response to methotrexate, and both were responsive to cisplatin-containing regimens. In contrast to the adenocarcinoma-like histological types, the squamous-like histological types did not respond as well to the anthracyclines.54 Overall response rates as high as 38% have been seen in mucoepidermoid carcinoma using non-anthracycline-containing chemotherapy combinations.55
Some newer agents hold promise for treating patients with advanced salivary gland carcinomas. In a small study of nine patients with adenocarcinoma-like tumor histological type, epirubicin combined with cisplatin and 5-FU demonstrated an overall response rate of 44.4%.48 Vinorelbine (Navelbine) appears to have single response rate activity in adenocarcinoma-like histological types, showing an overall response rate of 20% in a small study of 20 patients.56 Another study randomized 36 patients with predominantly adenocarcinoma-like tumor histological type to receive single-agent vinorelbine versus vinorelbine combined with cisplatin. The combination arm was superior, showing an overall response rate of 44% compared with 20% with the single agent.57 Finally, the taxanes appear to have activity in salivary gland carcinomas. A small study of 14 patients with advanced salivary gland carcinoma showed a response rate of 14% to carboplatin and paclitaxel.58 Other authors have reported success with this combination in patients with heavily pretreated metastatic disease.47
Acknowledgments Thanks to Maura Carroll for her expertise and assistance with word processing and typing, and to Patrick Wilson, M. D., for providing the photomicrographs for this chapter.
REFERENCES
1. Jaehne, M Ubmuller, J Jakel, KT, et al. The clinical presentations of non-Hodgkin’s lymphomas of the major salivary glands. Acta Otolaryngol 2001; 121: 647–651
2. Takahashi, H Tsuda, N Tezuka, F, et al. Non-Hodgkin’s lymphoma of the major salivary gland: a morphologic and immunohistochemical study of fifteen cases. J Oral Pathol Med 1990; 19: 306–312
3. Biasi, D Caramaschi, P Ambrosetti, A, et al. Mucosa-associated lymphoid tissue lymphoma of the salivary glands occurring in patients affected by Sjögren’s syndrome: report of six cases. Acta Haematol 2001; 105: 83–88
4. Harris, NL. Lymphoid proliferations of the salivary glands. Am J Clin Pathol 1999; 111 (1): S94–S103
5. Yuen, A Jacobs, C. Lymphomas of the head and neck. Semin Oncol 1999; 26 (3): 338–345
6. Allen, E Ali, S Matthew, S. Lymphoid lesions of the parotid. Diagn Cytopathol 1999; 21 (3): 170–173
7. Gleeson, MJ Bennett, MH Clawson, RA. Lymphomas of salivary glands. Cancer 1986; 58: 699–704
7a. Isaacson, P Wright, DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer 1983; 52 (8): 1410–1416.
8. Barnes, L Myers, EN Prokopakis, EP. Primary malignant lymphoma of the parotid gland. Arch Otolaryngol Head Neck Surg 1998; 124: 573–577
9. Hyman, GA Wolff, M. Malignant lymphomas of the salivary glands. Am J Clin Pathol 1976; 65: 421–438
10. Kojima, M Nakamura, S Ichimura, K, et al. Follicular lymphoma of the salivary gland: a clinicopathological and gastrointestinal lowgrade mucosa associated lymphoid tissue lymphoma—an analysis of seventy-five patients. J Clin Oncol 1999; 17 (4): 1254–1258
11. Ochoa, ER Harris, NL Pilch, BZ. Marginal zone B-cell lymphoma of the salivary gland arising in chronic sclerosing sialadenitis (Kuttner tumor). Am J Surg Pathol 2001; 25 (12): 1546–1550
12. Jaffe, ES. Lymphoid lesions of the head and neck: a model of lymphocyte homing and lymphogenesis. Mod Pathol. 2002; 15 (3): 255–263
13. Hyjek, E Smith, WJ Isaacson, PJ. Primary B cell lymphoma of salivary glands and its relationship to myoepithelial sialadenitis. Hum Pathol 1988; 19 (7): 766–776
14. Gasparotto, D Devita, S DeRe, V, et al. Extra-salivary lymphoma development in Sjögren’s syndrome: clonal evolution from parotid gland lymphoproliferation and role of local triggering. Arthritis Rheum 2003; 48 (11): 3181–3186
15. Nishimura, M Miyajima, S Okada, N. Salivary gland MALT lymphoma associated with Helicobacter pylori infection in a patient with Sjögren’s syndrome. J Dermatol 2000; 27: 450–452
16. Li, C Musich, PR Ha, T, et al. High prevalence of Helicobacter pylori in saliva demonstrated by a novel PCR assay. J Clin Pathol 1995; 48: 662–666
17. Jordan, RC Diss, C Millson, C Wilson, M Speight, PM. Absence of Helicobacter pylori DNA in salivary lymphoepithelial lesions. J Oral Pathol Med 1997; 26: 454–457
18. Alkan, S Karcher, DS Newman, MA Cohen, P. Regression of salivary gland MALT lymphoma after treatment for Helicobacter pylori. Lancet 1996; 348: 268–269
19. Wolvius, EB Jiwa, NM Vandervalk, P Horstman, A Vanderwall, I. Adenolymphoma and non-Hodgkin’s lymphoma of the salivary glands and oral cavity in immunocompetent patients are not associated with latent Epstein-Barr virus. Oral Oncol 1997; 33 (2): 119–123
20. Atula, T Grenman, R Klemi, P Syrjanen, S. Human papilloma virus, Epstein-Barr virus, human herpes virus 8, and human cytomegalovirus involvement in salivary gland tumors. Oral Oncol 1998; 34: 391–395
21. Diss, TC Wotherspood, AC Speight, P Pan, L Isaacon, PG. B cell monoclonality, Epstein-Barr virus and translocation in t(14;18) in myoepithelial sialadenitis and low-grade B cell and MALT lymphoma of the parotid gland. Am J Surg Pathol. 1995; 19 (5): 531–536
22. Hew, WS Carey, FA Kernohan, NM, et al. Primary T-cell lymphoma of salivary gland: report of a case and review of the literature. J Clin Pathol 2002; 55 (1): 61–63
23. Hsi, ED Singleton, TP Swinnen, L Dunphy, C Alkin, S. Mucosa associated lymphoid tissue type lymphomas occurring in posttransplantation patients. Am J Surg Pathol 2000; 24 (1): 100–115
24. DiGiuseppe, JA Corio, RL Wester, WH. Lymphoid infiltrates of the salivary glands: pathology, biology and clinical significance. Curr Opin Oncol 1996; 8 (3): 232–237
25. Ioachim, HL Antonescu, C Giancotti, F Dorsett, B. EBV-associated primary lymphomas in salivary glands of HIV-infected patients. Pathol Res Pract 1998; 194: 87–95
26. Joshi, VV Gagnon, GA Chadwick, EG, et al. The spectrum of mucosa-associated lymphoid tissue lesions in pediatric patients infected with HIV: a clinicopathologic study of six cases. Am J Clin Pathol 1997; 107 (5): 592–600
27. Zinzani, PL Magagnoli, M Galieni, P, et al. Non-gastrointestinal lowgrade mucosa associated lymphoid tissue lymphoma: an analysis of seventy-five patients. J Clin Oncol 1999; 17 (4): 1254–1258
28. De Rev, V De Vita, S Gasparotto, D, et al. Salivary gland B-cell lymphoproliferative disorders in Sjögren syndrome present a restricted use of antigen receptor gene segments similar to those used by hepatitis C virus–associated non-Hodgkin’s lymphomas. Eur J Immunol 2002; 32: 903–910
29. Klussmann, JP Muller, A Wagner, M Guntinas-Lichius, O, et al. Human herpes virus, type 8 in salivary gland tumors. J Clin Virol 2000; 16: 239–246
30. Klussmann, JP Wagner, M Guntinas-Lichius, O Muller, A. Detection of HHV-8 sequences and antigens in a MALT lymphoma associated with Sjögren’s syndrome. J Oral Pathol Med 2003; 32: 243–245
31. Isaacson, PG Spencer, J. Malignant lymphoma of mucosa associated lymphoid tissue. Histopathology 1987; 11: 445–462
32. Horning, SJ. Purine analogs in marginal zone lymphomas. Ann Oncol 1996; 7 (6): S21–S26
33. Shi, Q Zhang, T Xue, Q, et al. Clinicopathologic study of mucosa associated lymphoid tissue lymphoma of the salivary gland. Chin Med J (Engl) 2001; 114 (1): 44–47
34. Abbondanzo, SL. Extranodal marginal zone B-cell lymphoma of the salivary gland. Ann Diagn Pathol 2001; 5 (4): 246–254
35. Chan, JK Tsang, WY Hui, PK, et al. T and T/natural killer cell lymphomas of the salivary gland: a clinicopathologic, immunohistochemical and molecular study of six cases. Hum Pathol 1997; 28 (2): 238–245
36. Yencha, MW. Primary parotid gland Hodgkin’s lymphoma. Ann Otol Rhinol Laryngol 2002; 111: 338–342
37. Ü stün, MO Ekinci, N Payzin, B. Extramedullary plasmacytoma of the parotid gland: report of a case with extensive amyloid deposition masking the cytologic and histologic picture. Acta Cytol 2001; 45 (3): 449–453
38. Kerr, PD Dort, JC. Primary extramedullary plasmacytoma of the salivary glands. J Laryngol Otol 1991; 105: 687–692
39. Ott, G Katzenberger, T Greiner, A, et al. The t(11;18) (Q21;Q21) chromosome translocation is a frequent and specific aberration in low-grade, but not high-grade malignancy non-Hodgkin’s lymphomas of the mucosa-associated lymphoid tissue (MALT) type. Cancer Res 1997; 57 (18): 3944–3948
40. Kalla, J Stilgenbauer, S Schaffner, C, et al. Heterogeneity of the AP12-MALT1 gene rearrangement in MALT-type lymphoma. Leukemia 2000; 14: 1967–1970
41. Kerrigan, DP Irons, J Chen, I. BCL-2 gene rearrangement in salivary gland lymphoma. Am J Surg Pathol 1990; 14 (12): 1133–1138
42. Pisa, EK Pisa, P Kang, H Fox, RI. High frequency of t(14;18) translocation in salivary gland lymphomas from Sjögren syndrome patients. J Exp Med 1991; 174: 1245–1250
43. Tsang, RW Gospodarowicz, MK Pintilie, M, et al. Stage I and II MALT lymphoma: results of treatment with radiotherapy. Int J Radiat Oncol Biol Phys 2001; 50 (5): 1258–1264
44. Malek, SN Hatfield, AJ Flinn, IW. MALT lymphomas. Curr Treat Options Oncol 2003; 4 (4): 269–279
45. Mehle, ME Kraus, DH Wood, BG, et al. Lymphoma of the parotid gland. Laryngoscope 1993; 103: 17–21
46. Jager, G Neumeister, P Brezinschek, R, et al. Treatment of extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type with cladribine: a phase II study. J Clin Oncol 2002; 20 (18): 3872–3873
47. Ruzich, JC Ciesla, MC Clark, JI. Response to paclitaxel and carboplatin in metastatic salivary gland cancer: a case report. Head Neck 2002; 24: 406–410
48. Airoldi, M Pedani, F Brando, V Gabriele, P Giordano, C. Cisplatin, epirubicin, and 5-fluorouracil combination chemotherapy for recurrent carcinoma of the salivary gland. Tumori 1989; 75: 252–256
49. Hill, ME Constenla, DO A’Hern, RP, et al. Cisplatin and 5- fluorouracil for symptom control in advanced salivary adenoid cystic carcinoma. Oral Oncol 1997; 33 (4): 275–278
50. Dimery, IW Legha, SS Shirinian, M Hong, WK. Fluorouracil, doxorubicin, cyclophosphamide and cisplatin combination chemotherapy in advanced or recurrent salivary gland carcinoma. J Clin Oncol 1990; 8 (6): 1056–1062
51. Licitra, L Marchini, S Spinazze, S, et al. Cisplatin in advanced salivary gland carcinoma: a phase II study of twenty-five patients. Cancer 1991; 68 (9): 1874–1877
52. Licitra, L Cavina, R Grandi, C, et al. Cisplatin, doxorubicin and cyclophosphamide in advanced salivary gland carcinoma: a phase II trial of twenty-two patients. Ann Oncol 1996; 7: 640–642
53. Suen, JY Johns, ME. Chemotherapy for salivary gland cancer. Laryngoscope 1982; 92: 235–239
54. Kaplan, MJ Johns, ME Cantrell, RW. Chemotherapy for salivary gland cancer. Otolaryngol Head Neck Surg 1986; 95: 165–170
55. Creagan, ET Woods, JE Rubin, J Schaid, DJ. Cisplatin-based chemotherapy for neoplasms arising from salivary glands and contiguous structures in the head and neck. Cancer 1988; 62: 2313–2319
56. Airoldi, M Bumma, C Bertetto, O Gabriele, P Succo, G Pedani, F. Vinorelbine treatment of salivary gland carcinomas. Bull Cancer 1998; 85 (10): 892–894
57. Airoldi, M Pedani, F Succo, G Gabriele, AM. Phase II randomized trial comparing vinorelbine vs. vinorelbine plus cisplatin in patients with recurrent salivary gland malignancies. Cancer 2001; 91 (3): 541–547
58. Airoldi, M Fornari, G Pedani, F, et al. Paclitaxel and carboplatin for recurrent salivary gland malignancies. Anticancer Res 2000; 20: 3781–3784
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


