
2

Imaging of the Salivary Glands
The imaging algorithm for assessing the salivary glands depends on the clinical scenario with which the patient presents to his or her physician. There is a spectrum of imaging studies that may be used to assess salivary gland pathology, including computed tomography (CT), magnetic resonance imaging (MRI), ultrasound (US), and sialography. However, CT and MRI have sensitivities over 95% in detecting masses of the salivary glands and have largely replaced sialography in the evaluation of salivary masses.1
Many of the disease processes that affect the salivary glands may not require imaging of any kind. Such processes include self-limited entities like viral parotitis, mumps, and sialosis. At the other end of the spectrum are the infiltrative, deep lobe parotid masses/malignancies that usually require cross-sectional imaging, including CT or MRI, to assess for perineural spread of disease, infiltration of the skull base, intracranial extension of disease, and/or vascular involvement. In Europe and Japan, in the hands of an experienced sonographer, ultrasound is frequently the first imaging modality used to assess superficial salivary gland lesions.2,3
Patients with salivary gland pathology may present clinically with a suspected mass or with suspected obstruction and/or inflammation (diffuse unilateral or bilateral glandular enlargement). Although there may be overlap with many of the clinical entities, the initial imaging study desired may change depending on each of these presentations. In the discussion that follows, the role of imaging in assessing salivary gland pathology will be addressed and will be divided into the evaluation of neoplastic lesions, obstructive/inflammatory lesions, and systemic disorders affecting the salivary glands.
 Neoplasms of the Salivary Glands
Neoplasms of the Salivary Glands
A painless mass is usually due to a neoplasm (benign or malignant), although neoplasms may occasionally present with dull pain and suggest an inflammatory process. In the parotid region, a painless mass may also be caused by intraparotid or periparotid lymph nodes or a parotid cyst.
The superficial layer of the deep cervical facial fascia encapsulates the parotid gland late in the second trimester of gestation; as a result, it is the only salivary gland to incorporate lymphatic tissue and lymph nodes.3,4 Therefore, the potential for lymphadenopathy in the parotid gland exists. It is important that the radiologist determine if the pathologic lymph nodes are within the parotid gland or extraparotid at the time of imaging. Malignant adenopathy is typically seen in the setting of dermatologic malignancies (basal cell carcinoma, melanoma, and squamous cell carcinoma) (Fig. 2–1).5,6 Occasionally, metastatic adenocarcinoma (breast, colon, lung) or metastatic squamous cell carcinoma from a primary aerodigestive tract malignancy may result in parotid lymphadenopathy.5,7 In addition, lymphoma may occur primarily in the parotid gland as a dominant mass or infiltrative lesion, or may secondarily affect the parotid gland in the setting of systemic disease.7
Bilateral parotid gland masses are usually due to lymphadenopathy or Warthin’s tumors and rarely acinic cell carcinoma.8 Human immunodeficiency virus (HIV) frequently has bilateral parotid masses corresponding to a combination of lymphoepithelial cysts and lymph nodes,9,10 as is also seen in Sjögren’s syndrome.11,12 Multiple painless masses within a single parotid gland may be caused by Warthin’s tumors,13 lymph nodes, and, less commonly, acinic cell carcinoma, metastatic disease,14 and oncocytomas.15
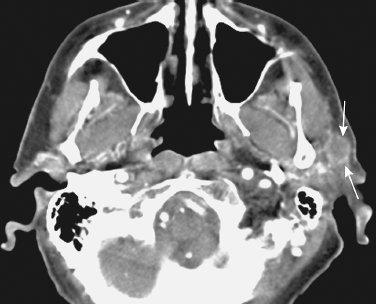
FIGURE 2-1 A 55-year-old male with a prior history of squamous cancer of the scalp presented with a new left cheek mass due to metastatic squamous cell carcinoma to an intraparotid lymph node. Axial contrast-enhanced computed tomography (CT) image shows an 8 mm mass (arrows) in the superficial left parotid gland.
Many would advocate MRI as the primary modality for assessing neoplasms of the salivary glands.16–19 If the referring clinician is highly confident that the glandular process is neoplastic, then MRI is the first, and frequently the only, imaging technique used. However, if there is even a slight chance that the mass could be related to sialadenitis or sialolithiasis, then unenhanced CT utilizing thin (1–3 mm) sections should be performed first, as MRI is not as sensitive or accurate in detecting small calculi (while over 90% of these are seen on CT).20–23
There are many reasons why MRI is the preferred modality in assessing the patient suspected of having a neoplastic lesion of the salivary glands. MRI, due to its multiplanar capabilities, as well as the multiple pulse sequences available to assess such lesions (unenhanced T1-weighted, fat-suppressed T2-weighted, and enhanced axial and coronal fat-suppressed T1-weighted), is the best modality to “map” the extent of neoplasm.24 Fat is hyperintense (bright) on T-1 weighted images, whereas tissue with abundant water content is hypointense (dark) on T1-weighted imaging and hyperintense on T2-weighted imaging. Gadolinium is an intravenous contrast agent with paramagnetic effects resulting in hyperintensity (“bright” signal) that is taken up to varying degrees by neoplasms and inflammatory processes depending on their cellularity and vascularity.
In adults, the parotid gland has a high fat content with numerous thin interstitial septae. The intraglandular ducts and facial nerve are usually not visualized on CT, but can be identified on MRI25,26 using special pulse sequences. Stensen’s duct is well seen usually only when dilated. The retromandibular vein and external carotid artery are noted within the gland posterior to the ascending ramus of the mandible. Almost all parotid lesions are readily identified on unenhanced T1-weighted MRI (Fig. 2–2, 2–3) as being hypointense (dark) relative to the intrinsic high signal intensity of the fat comprising a large portion of the normal parotid gland.16,18,27 To confirm the presence of a parotid mass, T1-weighted images are most sensitive.18 One cannot rely on T2-weighted images alone, as some lesions (including some Warthin’s tumors and carcinomas) are similar in signal to the normal gland on this pulse sequence.18 Unenhanced T1-weighted images are useful for assessing the extent of neoplasm within the parotid gland itself (including superficial and deep lobe involvement) (Fig. 2–4). In addition, unenhanced T1-weighted images are useful in assessing for invasion of the adjacent bone (the ascending ramus of the mandible, mastoid tip, and skull base marrow) because in the setting of neoplastic infiltration in these locations, the normal hyperintense high signal intensity fat is replaced with hypointense/low signal intensity tissue (usually reflecting a combination of water and pathologic cells) (Fig. 2–4).28 On fat-suppressed gadolinium-enhanced T1-weighted images, the normal fat-containing bone marrow of the mandible and skull base should be hypointense (suppressed); however, in the setting of neoplastic infiltration, enhancing (hyperintense) tissue replacing this normal hypointense background is noted. Similarly, in the presence of perineural spread of disease, one should see replacement of the normal high signal intensity fat in the marrow and the skull base foramina on unenhanced T1-weighted images. Corresponding hyperintense enhancing tissue on the gadolinium-enhanced T1-weighted images is indicative of neoplastic infiltration (Fig. 2–5).8,24,29 In particular, the foramina must be closely assessed by the radiologist in the setting of malignant neoplasms of the parotid gland, including the stylomastoid foramen (seventh cranial nerve, CN VII), foramen ovale (CN V3), and foramen rotundum (CN V2). Finally, MRI is the most accurate imaging modality in detecting intracranial spread of disease, including involvement of the leptomeninges.30–32 MRI is accurate in demonstrating perineural, vascular, and dural invasion that may be present with parotid malignancies, particularly adenoid cystic carcinoma.31
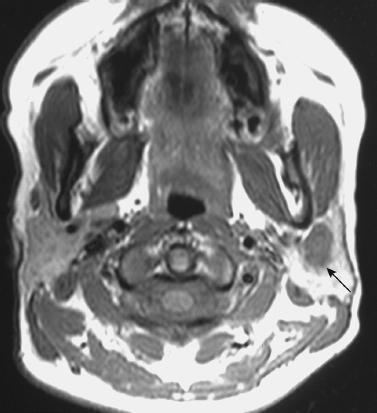
FIGURE 2-2 A 27-year-old woman with an incidental left parotid mixed tumor, detected on a brain magnetic resonance imaging (MRI) study, that was aspirated using CT guidance. Unenhanced axial T1-weighted MRI shows 1.2 cm hypointense mass of the left parotid gland (arrow). Note the hyperintense signal of the surrounding normal parotid gland due to its fat content.
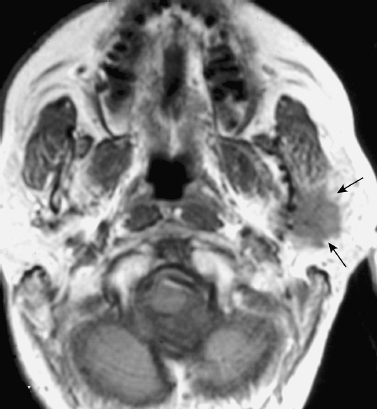
FIGURE 2-3 High-grade mucoepidermoid carcinoma of the left parotid gland. Axial unenhanced T1-weighted MRI shows a poorly demarcated hypointense mass (arrows).
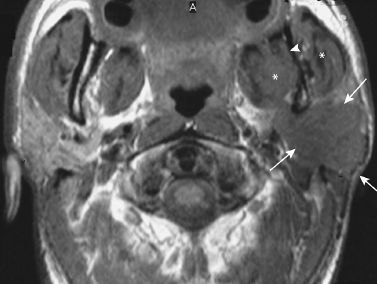
FIGURE 2-4 MRI of adenocarcinoma of the parotid gland with extensive extraglandular spread. Axial unenhanced T1-weighted MRI shows a poorly demarcated hypointense mass involving the superficial and deep lobes of the left parotid gland (arrows). There is spread to the mandibular foramen (arrowhead) and the masticator space (*).
In many cases, discrimination between benign and malignant tumors is not possible on MRI. There are studies suggesting that the presence of poorly defined tumor margins may best suggest the presence of malignancy.33 However, tumor margins and inhomogeneity cannot reliably predict benign from malignant disease (Fig. 2–6).16 Therefore, it may not be possible for the radiologist to distinguish benign from malignant disease in the setting of an isolated, contained intraparotid mass. The presence of infiltration into the adjacent extramucosal spaces, including the masticator space, parapharyngeal space, and the adjacent skull base, is highly suggestive of a malignant lesion.8 Furthermore, the presence of regional pathologic lymphadenopathy or perineural spread of disease is also usually indicative of neoplasm.17,34
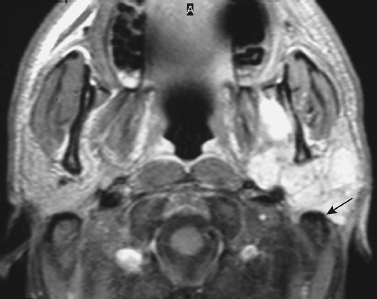
FIGURE 2-5 Enhanced axial T1-weighted MRI shows enhancement of the tumor that abuts the mastoid tip (arrow).
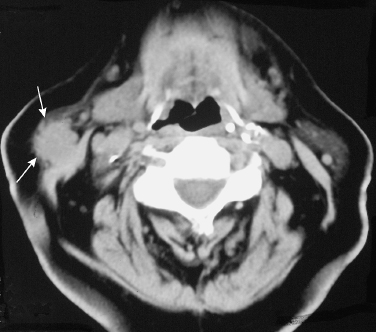
FIGURE 2-6 Axial CT image shows a poorly demarcated, spiculated mass in the right parotid tail (arrows) that represented a benign mixed tumor.
T2-weighted MR images tend to be most reliable (~75%) in suggesting the possibility of malignant disease.18 Typically, a lesion that is hyperintense on T2-weighted images is benign (Fig. 2–7), whereas a mass of intermediate or low signal intensity on T2-weighted images is of concern for malignancy (Fig. 2–8).18,27 This is because highly cellular tumors with a high nuclearto-cytoplasmic ratio (usually high-grade malignancies) frequently are intermediate to hypointense (dark) on T2-weighted images, whereas the less cellular, differentiated masses (benign tumors and low-grade malignancies) tend to be hyperintense on T2-weighted imaging due to higher water content.27 Most high-grade mucoepidermoid carcinomas (Fig. 2–8), undifferentiated carcinomas, adenoid cystic carcinomas, and occasional squamous cell carcinomas of the salivary glands demonstrate intermediate to low signal intensity on T2weighted images.18,27 However, some malignancies do exhibit increased signal intensity on T2-weighted images, including low-grade mucoepidermoid carcinomas, some adenoid cystic carcinomas, and, rarely, adenocarcinomas.16 It is also important to emphasize that pleomorphic adenomas may be hypointense on T2-weighted imaging, especially when cellular. Because they are far and away the most common parotid neoplasm, they will account for a significant number of the masses appearing dark on T2-weighted images; however, a malignant mass should always be excluded.
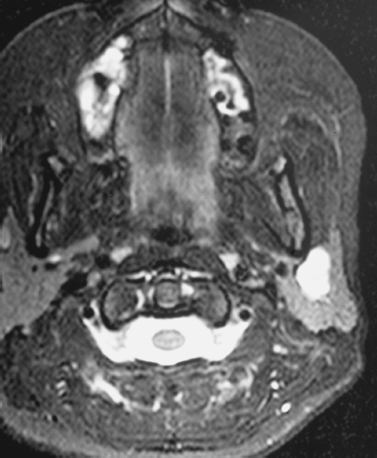
FIGURE 2-7 Same patient as in Fig. 2–2. Axial T2-weighted MRI shows the mass to be well demarcated and homogeneously hyperintense, typical of a mixed benign tumor.
The benign mixed tumor, like most benign tumors, is typically hyperintense on T2-weighted images (Fig. 2–7) due to extracellular water content, as well as intracellular water content due to a relatively low cellular-to-cytoplasmic ratio.8,18,27 Among the benign masses that may not always be hyperintense on T2weighted images are the cellular (less myxoid) mixed tumor and Warthin’s tumor.6 Therefore, a low signal intensity mass on T2-weighted imaging is not specific for a high-grade malignancy, but malignancy must be excluded. Other disease processes, including crystal deposition, fibrosis, and granulomas, can also be relatively T2-hypointense. In addition, chronic sialadenitis may appear hypointense on T2-weighted images.27 This variability has led some to suggest that the signal intensity on T2-weighted images is not of significant value.16
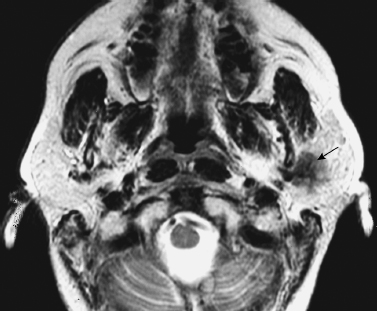
FIGURE 2-8 Same patient as in Fig. 2–3. Corresponding axial T2-weighted image shows that the mass is hypointense (arrow).
Caution is required in differentiating purely cystic lesions from tumors with solid and cystic components. Warthin’s tumors are typically complex lesions on T1-and T2-weighted images, containing areas that are solid and cystic.35,36 Septae are not uncommon, and they are frequently lobular (Fig. 2–9) One important imaging differential is the presence of T1-hyperintensity on unenhanced imaging corresponding to proteinaceous cysts with cholesterol crystals or hemorrhage.27 Fluid with a high protein concentration, as seen in the presence of blood products or mucoid material, may be hyperintense relative to normal cerebrospinal fluid.35 However, the signal characteristics of the fluid, especially on unenhanced T1-weighted imaging, will depend on the fluid’s viscosity and protein concentration. Simple fluid is usually hypointense on T1-weighted images and hyperintense on T2-weighted images.15 Benign cysts (including lymphoepithelial cyst, mucus retention cyst, sialocele, first branchial cleft cyst, and ranula) are also commonly hyperintense on T2-weighted images. Administration of contrast is essential to distinguish a cyst from a mass. Specifically, a cyst typically demonstrates rim enhancement, whereas a mass typically demonstrates more solid enhancement.15 It is important to note that some lesions that appear as simple cysts on unenhanced T1-weighted and T2weighted images may demonstrate solid enhancement following contrast administration, reflecting that these are not cysts at all, but rather neoplasms24 (Fig. 2–10). The value of contrast enhancement also applies to CT imaging (Fig. 2–11).
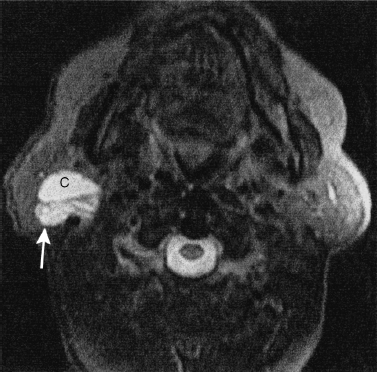
FIGURE 2-9 MRI of a complex cystic and solid Warthin’s tumor in the right parotid gland. Axial T2-weighted MRI shows the cystic (c) component of the mass that is homogeneously hyperintense (similar to cerebral spinal fluid), and the more solid component posteriorly (arrow). Also, note the septations within the tumor.
In the patient with prior parotid surgery for a malignant neoplasm and postoperative irradiation, fibrosis may be difficult to distinguish from recurrent neoplasm, as both are hypointense on T1-and T2-weighted images. In this scenario, positron emission tomography (PET) and/or imaging guided fine-needle aspiration (FNA) or biopsy may be indicated.29 In the follow-up imaging evaluation of patients operated on for mixed benign tumors, MR is clearly the imaging modality of choice as recurrent pleomorphic adenomas have a somewhat characteristic appearance, typically there are multiple T2-hyperintense cyst-like nodules (Fig. 2–12).
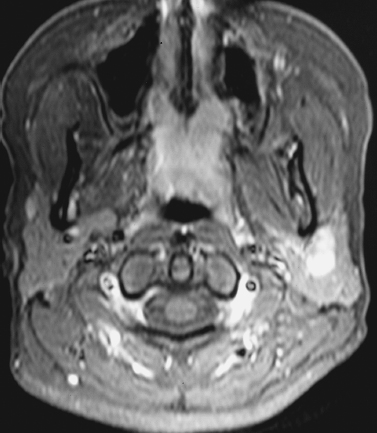
FIGURE 2-10 Same patient as in Fig. 2–2. Axial contrastenhanced fat-suppressed T1-weighted MRI shows that the mass homogeneously enhances
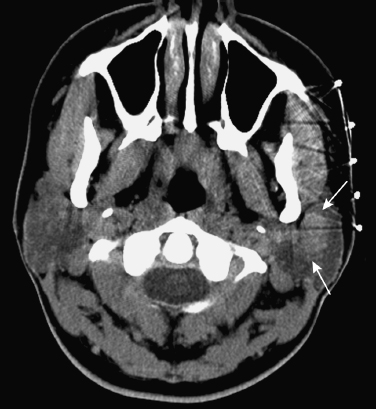
FIGURE 2-11 Same patient as in Fig. 2–2. Delayed enhanced axial CT image obtained approximately 6 minutes after injection shows the mass (arrows) is now clearly identified. For reasons not clearly understood, benign mixed tumors are frequently best delineated on CT using delayed imaging.
The attenuation of parotid masses on CT imaging allows one to distinguish a benign cyst from a solid mass, and distinguishes lipomas, but otherwise does not help in predicting histological diagnosis because most solid masses have a similar appearance on CT imaging.3,15 Because the appearance of a mass on CT is not a good predictor of histological diagnosis,37 and because MR imaging is more accurate than CT imaging in determining the extent of disease,38 MR imaging is advocated as the best imaging modality in the clinical setting of a suspected salivary gland mass.5 Computed tomographic images are also more susceptible to degradation by dental artifact.7 Although bone marrow involvement is better demonstrated on MR imaging, early cortical involvement of the mandible or skull base (mastoid tip) is best visualized on CT. Because neither study is histologically specific, FNA or biopsy is frequently performed to establish a histological diagnosis necessary to plan therapy.5,15,32
Nuclear scintigraphy may be useful in diagnosing Warthin’s tumors and oncocytomas. These neoplasms are unique in that they show increased radiotracer uptake on technetium Tc 99m pertechnetate imaging.1 Because these lesions are not associated with significant malignant potential, in patients who are elderly or who have surgical contraindications, observation may be advocated. Positron emission tomography (PET) has not been a reliable predictor of histological diagnosis, with an accuracy rate of ~60–70%.39
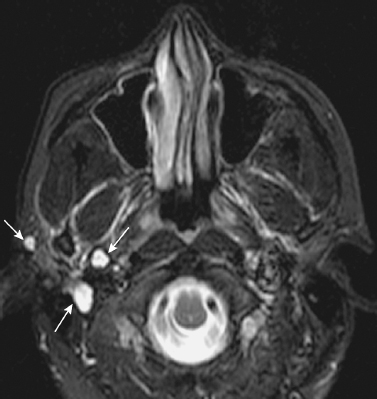
FIGURE 2-12 Recurrent pleomorphic adenoma 3.5 years following initial parotidectomy. Axial T2-weighted image shows the characteristic appearance of recurrent tumor, with multiple T2-hyperintense cystic-appearing nodules (arrows).
In Europe and Japan, ultrasound is frequently the first imaging technique utilized in assessing suspected masses within the superficial aspect of the parotid gland. Ultrasound can delineate the retromandibular vein and superficial temporal vein, but not the facial nerve. It is not accurate in assessing deep lobe parotid masses. In skilled hands, ultrasound can analyze superficial salivary gland neoplasms with an accuracy similar to CT and MR imaging.2,40 Gritzmann in a large series demonstrated that ~95% of space occupying major salivary gland tumors could be completely delineated on ultrasound.41 All neoplasms were hypoechoic relative to the normal hyperechoic fatty glandular tissue; however, ultrasound, like CT and MR, is not always accurate in distinguishing benign from malignant pathology. Ultrasound differentiated extraglandular from intragrandular lesions with 98% accuracy42 (all misinterpretations were periparotid lymph nodes).
Color doppler ultrasound may also be useful, as malignant salivary gland neoplasms frequently show a higher grade of vascularity compared with benign neoplasms.43 Flow alterations in color doppler ultrasound can be a physiologic parameter in disease states such as Sjögren’s.
The limitations of ultrasound even in skilled hands include its inability to assess deep parotid masses, obscuration of lesions by the mandible, and poor identification of extra-parotid spread of disease into the extramucosal spaces of the head and neck (parapharyngeal and retropharnygeal spaces).2 In addition, ultrasound is not good in assessing for perineural spread of disease or intracranial extension. Magnetic resonance and CT imaging have supplanted ultrasound in the evaluation of a salivary gland mass when such extension is suspected.3,44 Ultrasound guided FNA may be utilized in the evaluation of a superficial parotid mass that is either not readily palpable, or has both a solid and cystic component.
There is controversy as to the reliable identification of the facial nerve below the skull base.25,26,45–47 A line connecting the lateral surface of the posterior belly of the digastric muscle and the lateral surface of the mandibular ascending ramus has been used to separate superficial (lateral to the facial nerve) from deep (medial to the facial nerve) parotid masses on imaging.26,48–50 The differentiation of deep from superficial parotid masses is critical as this will dictate the extent of dissection needed to separate the nerve from the tumor, the attendant risk to the facial nerve, and in the case of tumors involving the parapharyngeal space, the need for a cervical approach with or without mandibulotomy.44,51,52
CT and MR imaging play an important role in assessing parapharyngeal space masses including deep lobe parotid tumors, minor salivary gland tumors, and schwannomas (Fig. 2–13, 2–14). Deep lobe parotid tumors and minor salivary gland tumors in the para-pharyngeal space lie in the pre-styloid compartment anterior to the carotid artery. Deep lobe parotid tumors are connected to the parotid and can displace the parapharyngeal fat medially. One of the largest issues the surgeon encounters is whether a lesion is arising from the deep lobe of the parotid gland or primarily from minor salivary gland tissue in the parapharyngeal space.53 With large lesions this can be difficult to determine with certainty. In the post-styloid compartment (the carotid space), common lesions include schwannomas and paragangliomas. They arise posterior to the internal carotid artery and displace it anteriorly. Schwannomas and paragangliomas enhance with gadolinium. Paragangliomas may be further characterized by the presence of serpintine flow voids (salt and pepper appearance) due to the marked vascularity that these lesions can have.
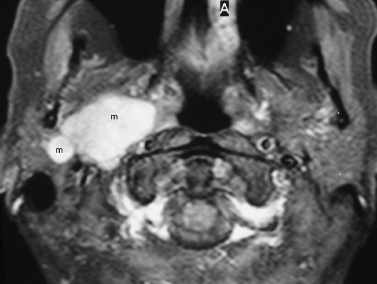
FIGURE 2-13 Mixed benign tumor of the deep lobe of the parotid gland. Axial enhanced fat-suppressed T1-weighted MRI shows a multilobular, enhancing mixed mass (m) of the deep lobe of the right parotid gland, extending to the prestyloid parapharyngeal space.
Increasingly, CT image-guided aspirations have become very useful in the assessment of a painless mass of the salivary glands. The most common request for CT-guided aspirations is in the assessment of deep lobe parotid or incidental parapharyngeal space masses (Fig. 2–15).54 Frequently, these lesions are not palpable and difficult to approach endoscopically.
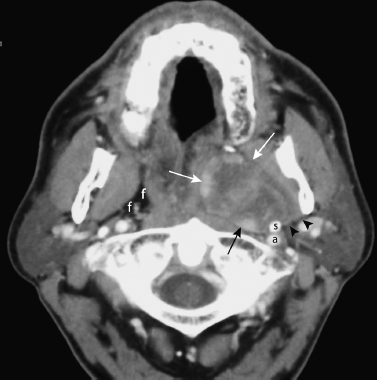
FIGURE 2-14 Schwannoma of the parapharyngeal space. Axial enhanced CT image obtained at the skull base shows a well-defined, heterogeneously enhancing mass (arrows) arising in the left parapharyngeal space. There is a fat plane (arrowheads) separating the mass from the deep lobe of the parotid gland. There is sclerosis of the styloid process (s), suggesting the mass has been there a long time. a, left internal carotid artery; f, fat comprising the normal right parapharyngeal space.
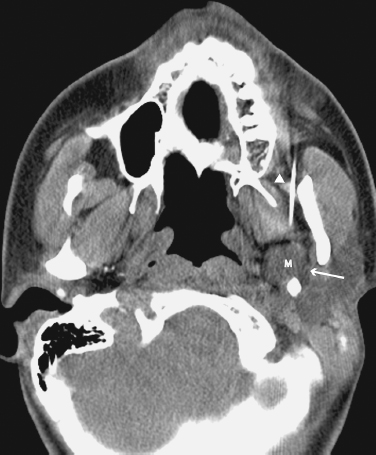
FIGURE 2-15 CT-guided fine-needle aspiration of a left parapharyngeal space mass; cytology revealed mixed benign tumor. Axial unenhanced CT image shows a 14 mm mass (m) in the left parapharyngeal space. Note the hypodense fat plane (arrow) separating the mass from the deep lobe of the parotid gland, and the needle (arrowhead).
 Obstructive or Inflammatory Lesions
Obstructive or Inflammatory Lesions
The classic signs of pain and swelling of the involved gland are typical in patients presenting with sialolithiasis (calculi of the salivary glands).20,55–57 Sialolithiasis is the second most common cause of salivary gland disease after viral infection or mumps. Typically the gland may be diffusely or focally enlarged with a sialolith in the proximal duct. Sialolithiasis most commonly affects the submandibular gland.40,58 Approximately 80% of sialoliths occur here because the saliva from this gland is more alkaline, thicker, and viscous. Calculi may be multiple (25%), and may occur within intraglandular ductal tributories or within the main ducts. When in the gland itself, the symptoms may be relatively minor, whereas a main ductal sialolith usually has a more acute presentation.
Imaging is extremely valuable in localizing sialoliths. Computed tomography,20 ultrasound,59,60 and plain film radiography accurately identify sialoliths (see Chapter 7). Most important is distinguishing intraductal versus intraglandular calculi. Ultrasound is less accurate than CT in distinguishing clusters of stones from single, large stones.57,60 In general, CT is the imaging modality of choice, and should be performed using thin section (1–3 mm) images. Intravenous contrast should not be administered as small opacified blood vessels may mimic small sialoliths. If an associated inflammatory process or infection is clinically suspected, then contrast enhanced imaging can be performed following unenhanced imaging.15
In recent years, some investigators have suggested fast T2-weighted MR imaging with thin sections to noninvasively evaluate ductal architecture and to identify stones.21,61Although the ductal system can be nicely demonstrated with thin section T2-weighted MR images, tiny intraglandular calculi, and stones within the main ducts can be overlooked with MR images because of the signal void associated with the calcified stone.61 In cases of a painful salivary gland associated with chronic sialadenitis without a calculus seen on CT, ultrasound, or plain film radiography, the appearance of the ductal system that MR imaging affords may provide information regarding the etiology of the painful gland. Strictures may be identified in the site of a prior sialolith. In other cases, sialadenitis may not be from calculi, but from other conditions such as autoimmune disease, and the ductal appearance may suggest the cause. For example, pruned or truncated main salivary ducts with globular collections within the peripheral gland parenchyma is typical for autoimmune inflammatory conditions.62,63 Large ducts are typically spared.
Sialography is contraindicated in the acute setting of suspected sialadenitis because of the possibility of exacerbating the symptoms associated with the infection.62 Retrograde injection of contrast material may force inflammatory products into the peripheral parenchyma of the gland. Furthermore, instrumentation may cause post-traumatic edema within the duct or stricture formation, leading to reduced drainage of the infected saliva.15 CT is more sensitive than sialography in demonstrating calculi. Sialography has been used because calculi rarely cause complete obstruction. A filling defect representing the calculus occurs as contrast flows around it. Chronic inflammatory lesions give similar sialographic change including segmental strictures and dilation, saccular dilation of the terminal ducts (punctuate, globular, cavitary, and destructive), and pseudocyst formation. Sialography remains of value in assessing penetrating trauma. Radiosialography using technetium has a limited role in assessment of parenchymal function.
Magnetic resonance sialography has the advantage of not requiring cannulation of the duct, and has a heightened sensitivity to edema in the salivary gland.64,65 However, in general, the need to perform conventional or MR sialography is limited to a handful of instances when clinical assessment, serology (autoimmune disease) conventional radiography, and/or CT cannot facilitate the diagnosis of chronic sialadenitis.
On cross-sectional imaging, an inflamed gland is usually enlarged, with abnormal attenuation or signal intensity, and prominent enhancement. There is frequently soft tissue stranding in the surrounding subcutaneous fat and tissues. The involved gland will be hyperintense on T2-weighted MR imaging. There may be adjacent enlarged lymph nodes or intraglandular lymph nodes in the setting of parotitis. Inclusion of CT and MR images in the coronal plane to assess inflammatory conditions of the parotid and submandibular glands may be valuable. The relationship of the inflammatory mass to the floor of the mouth for submandibular lesions, and the skull base for parotid gland lesions, is important regarding surgical approach.
A ranula can be completely characterized regarding its relationship to the mylohyoid muscle allowing the surgeons to determine surgical approach.15 A ranula that plunges through the muscular floor (a plunging ranula or pseudocyst, not epithelial lined) may be excised through a transcervical, submandibular incision.
 Systemic Diseases
Systemic Diseases
There are numerous imaging manifestations of chronic sialadenitis, most importantly chronic sialolithiasis may result in a small atrophic gland with focal intraglandular calcifications. Systemic disorders that may affect the major salivary glands include autoimmune disease, HIV infection, Sjögren’s, and sarcoidosis. CT may be the best way to image these patients because calculi may be responsible for the acute symptoms related to the systemic disorder. Sjögren’s syndrome and sarcoidosis predispose patients to stone formation (Fig. 2–16). The attenuation within the gland may be increased with both Sjogren’s disease and sarcoidosis. Focal masses may be present including cysts, nodules, and lymph nodes in all of the autoimmune diseases. In most cases, the diagnosis of Sjogren’s disease; however, can be made clinically based on the sicca syndrome and the connective tissue disorder (i. e., rheumatoid arthritis) combined with serology of antinuclear antibodies. Sialography may occasionally be a value in staging Sjogren’s syndrome. Punctate, globular, and destructive glandular ductal patterns may be discerned with MR sialography.15 There is an increased risk of lymphoma within the parotid gland in patients with Sjögren’s syndrome by more than 4,000%.66 Any dominant parotid mass in a patient with Sjögren’s syndrome should be considered to be lymphoma and requires fine-needle aspiration or biopsy. MR imaging may be particularly useful in discerning cysts from dominant masses within the parotid glands.66
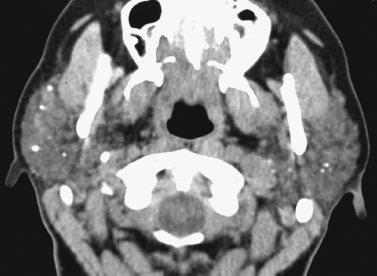
FIGURE 2-16 Axial enhanced CT image shows chronic sialadenitis in a patient with Sjögren’s syndrome. Note the increased density/attenuation of the bilateral parotid glands, as well as multiple, tiny, punctuate intraglandular calcifications/calculi.
Som et al used the phrase “acquired immunodeficiency syndrome-related parotid cyst” to describe the cysts associated with HIV infection that have a similar appearance to Sjogren’s related benign lymphoepithelial lesions.11 In HIV infection, cysts and lymphoid nodules/lymph nodes may be present in the parotid glands (Fig. 2–17). Additional findings include cervical lymphadenopathy, as well as hypertrophy of the adenoidal tissue.51,67
Sialosis refers to bilateral, painless enlargement of the salivary glands caused by systemic disorders such as diabetes mellitus, hyperthyroidism, alcoholism, and malnutrition. Certain medications have also been associated with sialosis.62 This disorder is rarely imaged but usually shows enlarged parotid glands with increased attenuation and slightly increased T2-weighted signal intensity. If there are calcifications within a painless parotid mass, benign mixed tumor, granulomatous disease, and occasional venous malformations are the most likely diagnoses.62 Sarcoidosis predisposes to glandular calcifications.
In summary, most disorders affecting the salivary glands will manifest with a few discrete clinical presentations that will guide imaging selection if necessary for further evaluation. Because sialolithiasis may have a spectrum of clinical manifestations, unenhanced CT should be the mainstay of imaging. Optimal imaging of the salivary glands may require unenhanced (to identify calculi), enhanced (to identify a mass), or both unenhanced and enhanced CT scans (for painful masses for which one cannot exclude sialolithiasis). However, for a nonpainful, noninflammatory mass in which there is a high degree of clinical suspicion for a neoplasm, contrast enhanced multiplanar MR imaging is the study of choice. Sialography, may occasionally be indicated and can be noninvasively performed with thin section heavily T2-weighted MR techniques. With CT, assessment of salivary gland tumors requires the administration of intravenous contrast to increase lesion conspicuity when there is a suspected neoplasm.
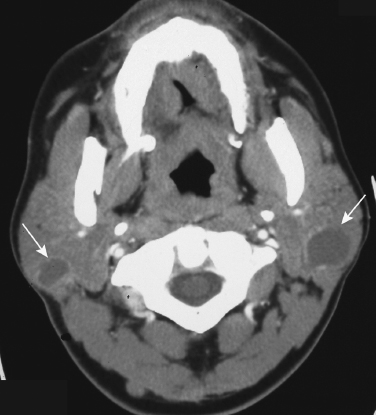
FIGURE 2-17 Axial enhanced CT scan shows lymphoepithelial cysts (arrows) in the bilateral parotid glands in this patient with human immunodeficiency virus (HIV) infection.
Imaging of the salivary glands has shifted away from predominantly utilizing plain films and conventional sialography to CT and MR imaging, as well as ultrasound.
REFERENCES
1. Calcaterra, T Hemenway, W Hansen, G Hanafee, W. The value of sialography in the diagnosis of parotid tumors. Arch Otolaryngol 1977; 103: 727–729
2. Wittich, GR Scheible, WF Hajek, PC. Ultrasonography of the salivary glands. Radiol Clin North Am 1985; 23: 29–37
3. Weissman, JL. Imaging of the salivary glands. Semin Ultrasound CT MR 1995; 16 (6): 546–568
4. Rabinov, KR Weber, AL. Radiology of the salivary glands. Boston: GK Hall; 1984
5. Tabor, EK Curtin, HD. MR of the salivary glands. Radiol Clin North Am 1989; 27 (2): 379–392
6. Soler, R Bargiela, A Requejo, I Rodriguez, E Rey, JL, Sancristan, F. Pictorial review: MR imaging of parotid tumours. Clin Radiol 1997; 52 (4): 269–275
7. Shah, GV. MR imaging of salivary glands. Magn Reson Imaging Clin N Am 2002; 10 (4): 631–662
8. Joe, VQ Westessan, PL. Tumours of the parotid gland: MR imaging characteristics of various histologic types. AJR Am J Roentgenol 1994; 163: 433–438
9. Shugar, JM Som, PM Jacobson, AL Ryan, JR Bernard, PJ Dickman, SH. Multicentric parotid cysts and cervical adenopathy in AIDS patients: a newly recognized entity—CT and MR manifestations. Laryngoscope 1988; 98: 772–775
10. Holliday, RA Cohen, WA Schinella, RA, et al. Benign lymphoepithelial parotid cysts and hyperplastic cervical adenopathy in AIDS-risk patients: a new CT appearance. Radiology 1988; 168: 439–441
11. Som, PM Brandwein, MS Silvers, A. Nodal inclusion cysts of the parotid gland and parapharyngeal space: a discussion of lymphoepithelial, AIDS-related parotid and branchial cysts, cystic Warthin’s tumors, and cysts in Sjögren’s syndrome. Laryngoscope 1995; 105: 1122–1128
12. Vogl, TJ Dresel, SH Grevers, G, et al. Sjögren’s syndrome: MR imaging of the parotid gland. Eur Radiol 1996; 6: 46–51
13. Shugar, JM Som, PM Biller, HF. Warthin’s tumor, a multifocal disease. Ann Otol Rhinol Laryngol 1982; 91: 246–249
14. Silvers, AR Som, PM. Salivary glands. Radiol Clin North Am 1998; 36: 941–966
15. Yousem, DM Kraut, MA Chalian, AA. Major salivary gland imaging. Radiology 2000; 216 (1): 19–29
16. Freling, NJ Molenaar, WM Vermey, A, et al. Malignant parotid tumors: clinical use of MR imaging and histologic correlation. Radiology 1992; 185: 691–696
17. Teresi, LM Lufkin, RB Wortham, DG Abemayor, E Hanafee, WN. Parotid masses: MR imaging. Radiology 1987; 163: 405–409
18. Schlakman, BN Yousem, DM. MR of intraparotid masses. AJNR Am J Neuroradiol 1993; 14: 1173–1180
19. Casselman, JW Mancuso, AA. Major salivary gland masses: comparison of MR imaging and CT. Radiology 1987; 165: 183–189
20. Avrahami, E Englender, M Chen, E Shabtay, D Katz, R Harell, M. CT of submandibular gland sialolithiasis. Neuroradiology 1996; 38: 287–290
21. Fischbach, R Kugel, H Ernst, S, et al. MR sialography: initial experience using a T2-weighted fast SE sequence. J Comput Assist Tomogr 1997; 21: 826–830
22. Murakami, R Baba, Y Nishimura, R, et al. MR sialography using half-Fourier acquisition single-shot turbo spin-echo (HASTE) sequences. AJNR Am J Neuroradiol 1998; 19: 959–961
23. Lomas, DJ Carroll, NR Johnson, G Antoun, NM Freer, CE. MR sialography: work in progress. Radiology 1996; 200: 129–133
24. Vogl, TJ Dresel, SH Späth, M, et al. Parotid gland: plain and gadolinium enhanced MR imaging. Radiology 1990; 177: 667–674
25. Dailiana, T Chakeres, D Schmalbrock, P Williams, P Aletras, A. High-resolution MR of the intraparotid facial nerve and parotid duct. AJNR Am J Neuroradiol 1997; 18: 165–172
26. Thibault, F Bonfils, P Halimi, P, et al. Is the facial nerve visible on magnetic resonance imaging? Ann Otolaryngol Chir Cervicofac 1992; 109: 365–368
27. Som, PM Biller, HF. High grade malignancies of the parotid gland: identification with MR imaging. Radiology 1989; 173: 823–826
28. Loevner, LA Tobey, JD Yousem, DM Sonners, AI Hsu, WC. MR characteristics of cranial marrow in adults with systemic disorders compared to normal controls. AJNR Am J Neuroradiol 2002; 23: 248–254
29. Barakos, JA Dillon, WP Chew, WM. Orbit, skull base, and pharynx: contrast-enhanced fat suppression MR imaging. Radiology 1991; 179: 191–198
30. Dillon, WP. Imaging of central nervous system tumors. Curr Opin Radiol 1991; 3: 46–50
31. Eisen, MD Yousem, DM Montone, KT, et al. Use of preoperative MR to predict dural, perineural, and venous sinus invasion of skull base tumors. AJNR Am J Neuroradiol 1996; 17: 1937–1945
32. Seethala, RR LiVolsi, VA Baloch, ZW. Relative accuracy of fineneedle aspiration and frozen section in the diagnosis of lesions of the parotid gland. Head Neck 2005; 27: 217–223
33. Raine, C Saliba, K Chippindale, AJ McLean, NR. Radiological imaging in primary parotid malignancy. Br J Plast Surg 2003; 56 (7): 637–643
34. Parker, GD Harnsberger, HR. Clinical-radiologic issues in perineural tumor spread of malignant diseases of the extracranial head and neck. Radiographics 1991; 11: 383–399
35. Swartz, JD Rothman, MI Marlowe, FI Berger, AS. MR imaging of parotid mass lesions: attempts at histopathologic differentiation. J Comput Assist Tomogr 1989; 13: 789–796
36. Minami, M Tanioka, H Oyama, K. Warthin tumour of the parotid gland: MR-pathological correlation. AJNR Am J Neuroradiol 1993; 14: 209–214
37. Berg, HM Jacobs, JB Kaufman, D Reede, DL. Correlation of fine needle aspiration biopsy and CT scanning of parotid masses. Laryngoscope 1986; 96: 1357–1362
38. Kaneda, T Minami, M Ozawa, K, et al. Imaging tumors of the minor salivary glands. Oral Surg Oral Med Oral Pathol 1994; 78: 385–390
39. Keyes, JW Jr Harkness, BA Greven, KM Williams, DW Watson, NE Jr. McGuirt WF. Salivary gland tumors: pretherapy evaluation with PET. Radiology 1994; 192: 99–102
40. Kress, E Schulz, HG Neumann, T. Diagnosis of diseases of the large salivary glands of the head by ultrasound, sialography and CT-sialography: a comparison of methods. HNO 1993; 41: 345–351
41. Gritzmann, N. Sonography of the salivary glands. AJR Am J Roentgenol 1989; 153: 161–166
42. Schroeder, HG Schwerk, WB Eichhorn, T. High-resolution realtime sonography in salivary gland diseases: II. Salivary gland tumors. HNO 1985; 33: 511–516
43. Martinoli, C Derchi, LE Solbiati, L Rizzatto, G Silvestri, E Giannoni, M. Color Doppler sonography of salivary glands. AJR Am J Roentgenol 1994; 163: 933–941
44. Howlett, DC Kesse, KW Hughes, DV Sallomi, DF. The role of imaging in the evaluation of parotid disease. Clin Radiol 2002; 57 (8): 692–701
45. Lufkin, R Teresi, L Wortham, D, et al. Magnetic resonance imaging of the facial nerve: normal anatomy and pathology. Acta Radiol Suppl 1986; 369: 212–214
46. Wortham, DG Teresi, LM Lufkin, RB Hanafee, WN Ward, PH. Magnetic resonance imaging of the facial nerve. Otolaryngol Head Neck Surg 1989; 101: 295–301
47. McGhee, RB Jr Chakeres, DW Schmalbrock, P Brogan, MA Negulesco, JA. The extracranial facial nerve: high resolution three-dimensional Fourier transform MR imaging. AJNR Am J Neuroradiol 1993; 14: 465–472
48. Eracleous, E Kallis, S Tziakouri, C Blease, S Gourtsoyiannis, N. Sonography, CT, CT sialography, MRI and MRI sialography in investigation of the facial nerve and the differentiation between deep and superficial parotid lesions. Neuroradiology 1997; 39: 506–511
49. Thibault, F Halimi, P Bely, N, et al. Internal architecture of the parotid gland at MR imaging: facial nerve or ductal system? Radiology 1993; 188: 701–705
50. Ariyoshi, Y Shimahara, M. Determining whether a parotid tumor is in the superficial or deep lobe using magnetic resonance imaging. J Oral Maxillofac Surg 1998; 56: 23–27
51. Shah, JP. Head and neck surgery, 2nd ed. London: Mosby-Wolfe; 1996
52. Sigal, R Monnet, O de Baere, T, et al. Adenoid cystic carcinoma of the head and neck: evaluation with MR imaging and clinicalpathologic correlation in 27 patients. Radiology 1992; 184: 95–101
53. Weissman, JL, Carrau, RL. Anterior facial vein and submandibular gland together: predicting the histology of submandibular masses with CT or MR imaging. Radiology 1998; 208: 441–446
54. Yousem, DM Sack, MJ Weinstein, GS Hayden, RE. Computer tomography–guided aspirations of parapharyngeal and skull base masses. Skull Base Surg 1995; 5: 131–136
55. Laudenbach, P. Salivary calculi. Rev Prat 1992; 42: 989–995
56. Haring, JI. Diagnosing salivary stones. J Am Dent Assoc 1991; 122: 75–76
57. Antoniadis, D Mendonidou, L Papanayotou, P Trigonidis, G. Clinical study of sialolithiasis: findings from 100 cases. Hell Stomatol Chron 1989; 33: 245–251
58. Levy, DM ReMine, WH Devine, KD. Salivary gland calculi. JAMA 1962; 181: 1115–1119
59. Murray, ME Buckenham, TM Joseph, AE. The role of ultrasound in screening patients referred for sialography: a possible protocol. Clin Otolaryngol Allied Sci 1996; 21: 21–23
60. Yoshimura, Y Inoue, Y Odagawa, T. Sonographic examination of sialolithiasis. J Oral Maxillofac Surg 1989; 47: 907–912
61. Varghese, JC Thornton, F Lucey, BC, et al. A prospective comparative study of MR sialography and conventional sialography of salivary duct disease. AJR Am J Roentgenol 1999; 173: 1497–1503
62. Silvers, AR Som, PM. Salivary glands. Radiol Clin North Am. 1998: 36 (5): 941–966
63. Som, PM Shugar, JM Train, JS Biller, HF. Manifestations of parotid gland enlargement: radiographic, pathologic, and clinical correlations: I. The autoimmune pseudosialectasias. Radiology 1981; 141: 415–419
64. Ohbayashi, N Yamada, I Yoshino, N Sasaki, T. Sjögren syndrome: comparison of assessments with MR sialography and conventional sialography. Radiology 1998; 209: 683–688
65. Tonami, H Ogawa, Y Matoba, M, et al. MR sialography in patients with Sjögren’s syndrome. AJNR Am J Neuroradiol 1998; 19: 1199–1203
66. Grevers, G Ihrler, S Vogl, TJ Weiss, M. A comparison of clinical, pathological and radiological findings with magnetic resonance imaging studies of lymphomas in patients with Sjögren’s syndrome. Eur Arch Otorhinolaryngol 1994; 251: 214–217
67. Yousem, DM Loevner, LA Tobey, JD Geckle, RJ Bilker, WB Chalian, AA. Adenoidal width and HIV factors. AJNR Am J Neuroradiol 1997; 18: 1721–1725
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


