Fig. 8.1
Pseudomembranous candidiasis in the soft palate
Erythematous candidiasis is characterized by unspecific focal or generalized redness of the mucous membranes (Fig. 8.2) and is often associated with symptoms as burning and stinging sensation. Antibiotics are the most common cause of acute primary erythematous candidiasis. Chronic secondary erythematous candidiasis is common in oral lichen planus.
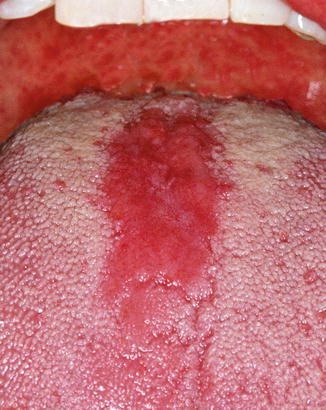

Fig. 8.2
Chronic erythematous candidiasis on the dorsal surface of the tongue (“median rhomboid glossitis”)
Hyperplastic candidiasis (Fig. 8.3) is a chronic infection and has two primary manifestations. The nodular type presents as small white slightly elevated papular lesions giving the mucous membrane a speckled appearance. The plaque-like type is homogenous white slightly elevated areas of the mucous membrane. It is often related to smoking and seen in the commissural area of the buccal mucosa. The hyperplastic lesions cannot be rubbed off and are usually asymptomatic. The two types may occur simultaneously. The hyperplastic types are to some extent controversial as they may represent secondary candida infections in leukoplakias (Holmstrup and Bessermann 1983), and the term candidal leukoplakia is sometimes used (Sitheeque and Samaranayake 2003). A biopsy is generally indicated to exclude malignancy, and the treatment result after antifungal therapy should always be monitored.
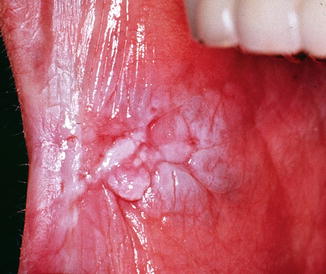

Fig. 8.3
Chronic hyperplastic candidiasis in the anterior part of the right buccal mucosa
Candida-associated lesions including denture stomatitis (Fig. 8.4), angular cheilitis, median rhomboid glossitis (Fig. 8.2) and linear gingival erythema may all be associated with Candida infection but may also be related to other causes, e.g. ill-filling dentures and bacterial or mixed bacterial and fungal infection. Thus, a thorough investigation is mandatory before adequate treatment can be initiated. In particular it is important to rule out a premalignant or malignant lesion, e.g. leukoplakia, erythroplakia or oral cancer, secondarily infected with Candida, or a disorder needing other management or supplementary treatment, e.g. lichen planus or lupus erythematosus (Table 8.1).
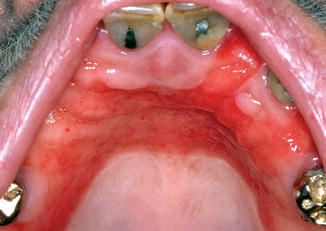

Fig. 8.4
Chronic erythematous candidiasis presenting as denture stomatitis
Table 8.1
Different types of oral candidiasis and differential diagnostic considerations
|
Oral candidiasis
|
Differential diagnostic considerations
|
|---|---|
|
Pseudomembranous
|
No similar lesion
|
|
Erythematous
|
Erythematous oral lichen planus, erythroplakia, oral cancer
|
|
Hyperplastic
|
|
|
Nodular
Plaque
|
Non-homogeneous leukoplakia, oral cancer
Homogeneous leukoplakia
|
|
Denture stomatitis
|
Ill-fitting dentures, poor denture hygiene
|
|
Angular cheilitis
|
Bacterial infection, malnutrition, vitamin and/or mineral deficiency
|
|
Median rhomboid glossitis
|
Geographic tongue, malnutrition, vitamin and/or mineral deficiency
|
|
Linear gingival erythema
|
Gingivitis (bacterial)
|
8.2.1 Histopathology
Candidal hyphae are readily identified in biopsies by the use of an appropriate staining method, e.g. Periodic Acid-Schiff (PAS) method or Grocott-Gomori methenamine silver (GMS) method. It should be mentioned that there is a risk of false-negative results if only few PAS-stained sections are examined (Roed-Petersen et al. 1970). Hyphae are seen in the parakeratin layer of the epithelium as a superficial infection (Fig. 8.5). Only in severely immunocompromised patients can hyphae be seen in the underlying epithelial layers or in the connective tissue. Typically, the epithelium is hyperplastic and hyperparakeratinized with leukocytes, in particular polymorphonuclear neutrophils, penetrating the epithelium often forming microabscesses in the superficial part of the epithelium in relation to the hyphae. Chronic inflammation is present in the connective tissue underneath the epithelium; however, this is often lacking in severely immunocompromised patients, e.g. patients with HIV infections and AIDS.
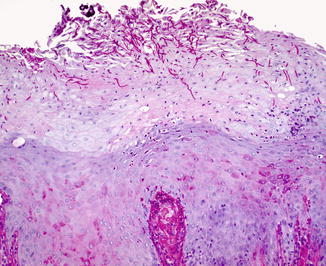

Fig. 8.5
Photomicrograph of a biopsy from a chronic hyperplastic candidiasis. Numerous PAS-positive (red) hyphae are seen in the parakeratin layer of the epithelium
8.2.2 Immunological Aspects
A large variety of proinflammatory and immunoregulatory cytokines are generated in the oral mucosa during an infection with Candida (Dongari-Bagtzoglou and Fidel 2005). It has been shown that highly invasive strains of C. albicans trigger production of proinflammatory cytokines, including IL-1α, IL-6, IL-8 and TNF-α in epithelial cells and IL-6, IL-8, monocyte chemotactic protein (MCP)-1, MCP-2 and granulocyte colony-stimulating factor in endothelial cells (Villar et al. 2005; Whiley et al. 2012).
8.3 Oral Candidiasis in the Medically Compromised Patient
Oral candidiasis is the result of yeast overgrowth and penetration of the epithelial mucosal protective barrier and is evident in patients with various conditions that exhibit immunosuppression including diabetes.
A large number of local and systemic factors and conditions may predispose individuals to oral candidiasis. Factors like treatment with antibiotics, ill-fitting dentures, poor oral hygiene and tobacco smoking can favour growth of yeast cells by disrupting the ecological balance on the mucosal surface (Baboni et al. 2009; Holmstrup and Bessermann 1983; Semlali et al. 2014). Treatment with immunosuppressives, cancer therapy, immune deficiencies, salivary gland hypofunction induced by medication or disease and diabetes can compromise local or general defence mechanisms and thereby lead to oral candidiasis. Often a patient will present several predisposing factors at the same time, and multiple interventions must be initiated including instruction, motivation and follow-up of oral hygiene procedures, diet change, stimulation of functional salivary glands, substitution of xerogenic drugs, diagnosis of oral mucosal diseases, tobacco counselling and smoking cessation and multidisciplinary diagnostic workup of systemic predispositions.
8.3.1 Oral Candidiasis as Adverse Drug Reaction
Systemic antibiotics are the most common cause of acute mucosal candidiasis. Oral exposure to topical glucocorticoid steroids is another common cause of medication-induced oral candidiasis, probably due to alterations of the local mucosal host immunity. Removal of the topical medication with water after exposure tends to prevent recurrence. General immune suppression due to systemic glucocorticoids, cancer chemotherapy and immunomodulators often causes mucosal candidiasis, which usually can be prevented by prophylactic antifungal treatment. Studies using both culture and pyrosequencing have shown that the oral mycobiota in immunosuppressed solid organ transplant recipients is dominated by Candida species (Charlson et al. 2012; Diaz et al. 2013; Dongari-Bagtzoglou et al. 2009). Salivary gland dysfunction resulting in reduced salivary flow rate (salivary hypofunction), compositional changes or a combination of both often has major consequences for the oral microbial balance and local immune defence leading to an increased risk of dental caries and oral candidiasis (Dawes et al. 2015). The main causes of salivary gland hypofunction are intake of medications, systemic diseases like Sjögren’s syndrome and head and neck radiotherapy (Villa et al. 2015; Jensen et al. 2010; Pedersen 2014).
8.3.2 Diabetes Mellitus and Oral Candidiasis
The carriage frequency of Candida and the density of candidal colonization as well as the rates of oral Candida infections are increased in patients with both type 1 and type 2 diabetes mellitus (DM) (Tapper-Jones et al. 1981; Lamey et al. 1988, 1992; Hill et al. 1989; Vazques and Sobel 1995; Bai et al. 1995; Guggenheimer et al. 2000; Kadir et al. 2002; Jurevic et al 2003; Shenoy et al. 2014). Oral candidiasis is not only more common in patients with DM than in nondiabetics, the infections are also more severe (Guggenheimer et al. 2000). The increased susceptibility to oral candidiasis has been related to poor glycaemic control and hence high concentrations of glucose in the blood and saliva, long disease duration as well as presence of diabetic complications (retinopathy) (Bai et al. 1995; Bartholomew et al. 1987; Dorocka-Bobkowska et al. 1996; Guggenheimer et al. 2000; Kadir et al. 2002; Ueta et al. 1993; Vazques and Sobel 1995). High concentrations of glucose in the blood and saliva may promote growth and enhance adherence of yeasts to epithelial cell surfaces (Samaranayake 1990). Also the impaired functions of polymorphonuclear leukocytes leading to reduced phagocytosis, intracellular killing and chemotaxis may contribute to the increased colonization of Candida and increased susceptibility to oral candidiasis (Ueta et al. 1993; Vazques and Sobel 1995). However, other risk factors such as salivary gland hypofunction, low salivary pH and impaired salivary antimicrobial activity, poor oral hygiene, cigarette smoking and denture wearing also have a substantial influence on candidal colonization and various oral manifestations and symptoms of Candida infections in both type 1 and 2 DM patients (Budtz-Jørgensen 1990; Banoczy et al. 1987; Guggenheimer et al. 2000; Jurevic et al. 2003; Kadir et al. 2002; Pedersen 2004; Samaranayake 1990; Willis et al. 1999). Inadequately controlled diabetics who wear dentures have a higher oral candida load and higher prevalence of denture stomatitis than nondiabetic denture wearers (Guggenheimer et al. 2000; Vitkov et al. 1999).
C. albicans is the most common species isolated from the oral cavity of diabetics (Dorocka-Bobkowska et al. 1996; Kadir et al. 2002; Samaranayake 1990; Willis et al. 1999), but also C. dubliniensis, C. glabrata and C. tropicalis have been isolated from the oral cavity of patients with diabetes (Jurevic et al. 2003). The significance of species in relation to pathogenesis of fungal infections in diabetics remains to be elucidated.
8.3.3 HIV Infection and Oral Candidiasis
Oral candidiasis is the most common opportunistic infection in HIV-infected patients and in patients with AIDS (Coleman et al. 1993; Shiboski et al. 2015) and one of the earliest indicators of the progression from HIV-seropositive status to AIDS. Oral candidiasis is strongly associated with immune suppression, as measured by CD4+ lymphocyte counts, and also associated with high viral burden and consequently suggested as a clinical marker of plasma viral load and the progression of HIV disease (Glick et al. 1994; Patton 2000).
The candida carriage in HIV-infected patients is predominated by C. albicans, but C. dubliniensis and C. glabrata have also commonly been isolated from oral lesions in HIV-infected patients (Sullivan et al. 1995; Li et al. 2007). The increased carriage of C. krusei has been associated with the widespread use of fluconazole prophylaxis (Samaranayke and Samaranayke 1994). A recent study on the oral mycobiome using pyrosequencing showed a shift in the oral mycobiome in which Epicoccum and Alternaria were abundantly colonizing HIV-infected patients, but Candida being abundant in both HIV patients and healthy subjects (Mukherjee et al. 2014).
The introduction of highly active antiretroviral therapy (HAART) has changed the epidemiology of oral candidiasis. Thus, several studies have reported a decrease in the prevalence and recurrence of oral candidiasis in HIV-infected patients receiving HAART (Greenspan et al. 2004; Jiang et al. 2014; Ramírez-Amador et al. 2007). However, some studies report rare cases of increased prevalence. There is substantial evidence suggesting that onset of oral candidiasis is associated with a progressive reduction in CD4+ lymphocyte count and an increase in viral load in HIV-infected patients receiving HAART (Hodgson et al. 2006; Ramírez-Amador et al. 2007). Oral candidiasis has therefore been suggested as a clinical marker of immune status and a predictor of virologic failure during HAART and hence an indicator of HIV disease progression and a tool for monitoring HIV infection in conjunction with CD4+ lymphocyte counts and plasma viral load in HIV-infected patients receiving HAART (Ramírez-Amador et al. 2007).
8.3.4 Recipients of Organ and Haematopoietic Cell Transplants and Cancer Therapy
Recipients of solid organ transplants and haematopoietic cell transplants and patients receiving cancer therapy have a high risk of developing fungal infections due to immunosuppressive, immunomodulating therapy as well as treatment with antibiotics (Trenschel et al. 2000). Despite antifungal prophylaxis, the increased risk for systemic and oropharyngeal fungal infection is still a matter of concern in these patients, and fungal infections remain a significant cause of morbidity and mortality. The prevalence of oral candidiasis in renal transplant recipients ranges from 9.4 to 46.7 % (Al-Mohaya et al. 2002; de la Rosa-García et al. 2005; Güleç et al. 2003).
C. albicans has been found to be the most prevalent species isolated from the oral cavity of recipients of kidney transplants (da Silva-Rocha et al. 2014). Also in recipients of liver transplants, the Candida carriage and prevalence of oral candidiasis are high (40–50 %), and many of these patients also suffer from salivary gland hypofunction (Helenius-Hietala et al. 2014).
The most common forms of oral candidiasis reported in patients receiving cancer therapy are pseudomembranous and erythematous candidiasis (Lalla et al. 2010). There appear to be no relation to Candida colonization and the presence or severity of oral mucositis in haemopoietic progenitor cell transplant patients (Epstein et al. 2003; Westbrook et al. 2013), whereas C. glabrata has been associated with oral ulcerations in this patient group (Laheij et al. 2012). A recent study indicates that the mycobiome plays a role in the pathogenesis of acute graft-versus-host disease in blood- and marrow-transplanted patients (van der Velden et al. 2013).
Several studies have shown that the oral microbiota is disturbed in patients, who has received radiotherapy in the head and neck region, and in this regard found increased colonization of Candida species and higher occurrence of oral candidiasis (Al-Nawas and Grötz 2006; Almståhl and Wikström 2003; Almståhl et al. 2008; Brown et al. 1975; Grötz et al. 2003). C. albicans is the cause of the majority of oropharyngeal infections, but C. glabrata and C. tropicalis are emerging causes of these infections in patients with head and neck cancer. It has been shown that about 50 % of patients with head and neck cancer were colonized with Candida species prior to radiotherapy, and after the therapy the percentage has increased to about 75 % (Lalla et al. 2010). The increased colonization also translated into an increased rate of oral infections. Predisposing factors in patients with head and neck cancer include mucosal injury due to cancer therapy, salivary gland hypofunction, smoking and wearing dentures.
8.4 Chronic Mucocutaneous Candidiasis
Chronic mucocutaneous candidiasis (CMC) is persistent or recurrent widespread superficial Candida infection of the oral, oesophageal, digestive, genital, nail mucous membranes and/or skin. Most often C. albicans is the infectious Candida species. CMC is caused by immune deficiencies involving the mucosal cutaneous immunity, which can have an inherited but most often have sporadic origin. T-cells seem to play an essential role, as several T-cell immune deficiencies, e.g. impaired T-cell function, activation and cytokine signalling, have been associated with CMC (Lanternier et al. 2013). Findings such as reduced proportion of interleukin-17 (IL-17)-secreting T-cells, autoantibodies directed against IL-17 and mutations in IL-17-related genes suggest that impairment of IL-17 immunity plays a significant role in CMC pathogenesis (Puel et al. 2012). CMC is associated with three syndromes including autosomal recessive autoimmune polyendocrinopathy syndrome type 1, hyper IgE syndrome and CARD9 deficiency (Al-Herz et al. 2011). CMC as the principal symptom or CMC in association to other skeletal, endocrine or skin abnormalities initiates in early childhood, and immune dysfunction should be suspected in patients without predisposing risk factors for oral candidiasis.
8.5 Diagnostic Methods
Identification of candidal overgrowth can be established by a variety of methods including culture, cytosmears, biopsy and molecular techniques (Table 8.2).
Table 8.2
Diagnostic methods, detection techniques and their advantages and disadvantages
|
Infection
|
Method
|
Traditional techniques
|
Time
(hours)
|
Traditional advantages
|
Molecular techniques
|
Time
(hours)
|
Molecular advantage
|
Disadvantages
|
|---|---|---|---|---|---|---|---|---|
|
Focal mucosal infection
|
Swab
Imprint
|
CFU
|
48
|
Quantification
Some species
identification
|
PCR/ MALDI-TOF
|
24–48
or
10 min.
|
Species
identification
|
No morphological
differentiation
Time
|
|
Cytosmear
|
PAS microscopy
|
~2
|
Quantification
Morphological differentiation
|
FISH
|
~2
|
Species identification
|
Expensive
|
|
|
Cytobrush
|
DNA
|
PCR
|
24–48
|
Species identification
|
No morphological
differentiation
No quantification
Time
|
|||
|
Biopsy
|
PAS microscopy
|
30
|
Some quantification
Morphological
differentiation
|
FISH
|
~2
|
Species
identification
|
Expensive
|
|
|
Generalized oral infection
|
Whole saliva
|
CFU
|
48
|
Quantification
Some species
identification
|
PCR/ MALDI-TOF
|
24–48
10 min.
|
Species
identification
|
No
morphological
differentiation
Time
|
|
Oral rinse
|
CFU
|
48
|
Quantification
Some species
identification
|
PCR/ MALDI-TOF
|
24–48
10 min.
|
Species
identification
|
No
morphological
differentiation
Time
|
8.5.1 Culture of Clinical Samples in Order to Quantify andIdentify the Candida Load
Different culture media can be used to grow and differentiate Candida spp. Some can easily be prepared, e.g. Sabouraud dextrose and Pagano-Levin agar media, and others are commercially available, e.g. CHROMagar™ (CHROMagar, France), chromID® Candida (BioMérieux, USA) and BiGGY Agar (Nickerson Agar) (Sigma-Aldrich®, USA). Up to four different Candida spp. can be differentiated by culture of clinical samples. Swaps, imprints, whole saliva and oral rinse can be the source for culture techniques. In immunocompetent patients, there is no universal arbitrary threshold level of colony-forming units (CFU) differentiating between carrier state and candidiasis as individual host and oral environmental factors influence the candida load (Epstein et al. 1980). However, in immunocompromised patients an arbitrary value of >400 CFU has been suggested for antimycotic intervention (Ship et al. 2007). Culture is time consuming and 48 h growth at 37 ° C delays the diagnosis. The composition of the Candida spp. in lesional infections may vary from the overall composition in the oral cavity, which make the choice of sampling procedure important (Kragelund et al. 2013).
8.5.2 Smears
Exfoliative cytologic examination is an easy and inexpensive method for detection of candida organisms. The suspected area is vigorously scraped with a wooden spatula and made into a smear on a glass microscope slide, spray fixed with a commercial spay or fixed in 70 % ethanol and stained appropriately, e.g. the PAS or GMS method. An advantage of this method compared to simple culture techniques is that the pathogenic form of the candida organism (hyphae) is easily identified. However, standardized culture of saliva or oral rinse samples is more suitable for identifying candida presence and load in the oral cavity.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


