
11

Metastasis to Major Salivary Glands
 Skin Cancer and the Salivary Glands
Skin Cancer and the Salivary Glands
Despite its prevalence, nonmelanoma skin cancer often manages to surprise both patients and clinicians with its destructive and sometimes lethal ability. The annual mortality rate from nonmelanotic cutaneous carcinoma approaches that for the more lethal but less common melanoma.1 As disfiguring as both forms of skin cancer can be, the mortality rates are mostly related to metastatic rather than local disease. Surgeons need to consider the pathways for metastases in melanoma and squamous cell skin cancer patients. Furthermore, only when the tumor is reliably extirpated should the surgeon focus on the reconstructive effort.
Skin cancer is the most common cancer in the world, and 80% of all skin cancers occur in the head and neck region.2 The incidence of these lesions by most accounts has doubled and tripled in the past several decades. Assessing and managing metastatic disease is complicated by the cranial nerves, particularly the facial nerve as it courses through the lymphatic-laden parotid gland.
Squamous cell carcinomas and melanomas comprise the overwhelming majority of head and neck malignant neoplasms that metastasize to the parotid;3 however, other tumors such as Merkel cell carcinoma, eccrine carcinomas, sebaceous carcinoma, olfactory neuroblastoma, retinoblastoma, thyroid, and intracranial neoplasms may be encountered. Merkel cell carcinoma and desmoplastic squamous cell carcinomas are of particular note for their metastatic potential. In patients with aggressive cutaneous carcinomas, the parotid gland will need to be addressed in the absence of clinically evident metastases.
Anatomy
The skin is an ectodermally derived three-layered structure consisting of the epidermis, the papillary dermis, and the reticular dermis. The latter rests upon a layer of fat that incorporates the deepest extension of hair follicles and eccrine sweat glands. Superficial skin cancers (those within the epidermis) can track unimpeded along hair follicles and sweat glands and persist after treatment if the treatment does not extend beneath the root of the follicle or sweat gland. Keratinocytes comprise the majority of the cells within the epidermis and give rise to basal and squamous cell carcinomas; however, the epidermis also contains melanocytes, Merkel cells, and Langerhans’ cells. Separating the keratinocytes from the papillary dermis is the basement membrane. Once a cancer cell violates the basement membrane, it has access to the lymphatic channels and the superficial vascular plexus, both of which reach into the papillary dermis. A fascial cover separates the parotid gland from the surrounding tissue. Extraglandular nodes are located in pretragal and supratragal tissues superficial to the posterior portion of the parotid and in the infra-auricular tissues superficial to the tail of the parotid. Metastatic involvement of these superficial nodes can lead to direct penetration of the parotid fascia, a particular potential for preauricular and auricular cutaneous carcinoma. A glandular infiltration of neoplasm has free reign of the gland and will simply push nerves, vessels, and nodes away as it expands. The facial nerve itself is often only infiltrated by an aggressive malignancy or an exceptionally large growth. Otherwise, the seventh nerve is “pushed” away from a growth; therefore, the nerve’s position cannot be protected without a dissection.
The parotid lymph nodes drain the face lateral to the nose and anterior to the ear. The scalp can be divided in a coronal plane through the external auditory canal with areas anterior to the plane draining into the parotid and submandibular nodes and areas posterior draining into the occipital nodes and posterior cervical nodes. The eyelids, conjunctiva, lacrimal gland, posterior cheek, upper neck, anterior ear, external auditory meatus, and eustachian tube drain to the parotid gland. The postauricular sulcus drains predominantly to the posterior neck (Fig. 11–1). The lymph nodes surrounding the submandibular gland are the focus of metastatic disease from the skin and mucous membranes of the anterior face, nose, lips, buccal mucosa, gingiva, mobile tongue, floor of the mouth, and palatoglossal folds.
Epidemiology of Skin Cancer
Each year approximately 500,000 people in the United States will be diagnosed with nonmelanoma skin cancer. Squamous cell carcinoma, comprising 20 to 30% of cutaneous malignancies, is second in incidence to basal cell carcinoma. Approximately 32,000 new cases of melanoma will be diagnosed each year. Ultraviolet radiation is the principal etiology4 of these cancers brought on by lifestyle choices and possibly ozone depletion.
Recent epidemiologic studies are revealing that skin cancer can be more than a disease unto itself; it can also be the harbinger of other cancers. Like the skin, the parotid gland is of ectodermal origin. Epidemiologic data have shown an increase in primary, nonmetastatic parotid cancer in patients with a prior history of skin cancer, suggesting an inherent susceptibility to ectodermal carcinogenesis in those individuals.5 In Switzerland, Levi et al5 found a threefold increase in the incidence of salivary gland cancers among patients with a history of basal cell carcinoma. They noted an even stronger association between squamous cell skin cancer and subsequent salivary gland cancers.6 In the United States, Kahn et al7 reported similar results and also looked at cancer mortality following a history of nonmelanoma skin cancer. Among the cancers contributing to an increased mortality in these patients were salivary gland carcinomas. The preceding three studies defined their cancers as second primaries and not metastatic lesions to the parotid. Speculation on the observed associations between nonmelanoma skin cancers and subsequent primary parotid neoplasms range from a common genetic susceptibility or environmental agent to a simple increase in encounters with the health care profession brought on by a disease (skin cancer) that the patient could not ignore.8
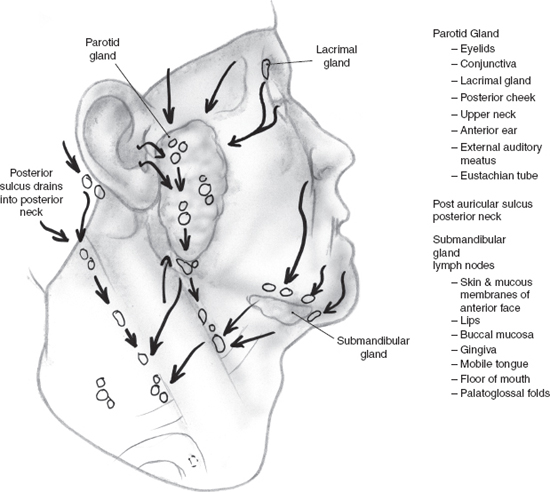
FIGURE 11-1 Lymphatic drainage to the salivary glands.
Evaluation and Treatment of the Skin Cancer Patient
A history and physical examination followed by a biopsy will initiate the evaluation of a skin cancer patient. Basal cell carcinomas and squamous cell carcinomas may present as nonhealing or flaking lesions. Physical examination should define the suspected size of the growth. The visible and microscopic extent of a nodular basal cell carcinoma rarely differs. However, poorly differentiated squamous cell carcinomas and morpheaform basal cell carcinomas are notorious for diving into tissue and extending beyond the reaches of the physician’s eye. Mohs micrographic surgery is the treatment of choice for the latter two types of skin cancers. Mohs could be considered for any nonmelanoma facial skin cancer. No published technique approaches the control offered by Mohs micrographic surgery for basal and squamous cell carcinomas. Many surgeons, including some Mohs surgeons, are reluctant to advocate Mohs surgery for all nonmelanoma head and neck skin cancers. Lack of access to a Mohs surgeon is a valid and frequently cited reason; however, the other frequently cited issue pertains to cost control and stems from a failure to recognize all of the health care costs involved in the care of a skin cancer patient when Mohs is not utilized. Cook and Zitelli9 effectively refute the notion that Mohs micrographic surgery is not cost-effective. They compared Mohs surgery to traditional excision with vertical section analysis and reviewed the costs to the health care system. Mohs did not increase costs, yet it indisputably increases local control. The increased use of Mohs surgery may diminish the incidence of recurrent and metastatic nonmelanotic cutaneous cancer. The use of Mohs surgery for melanoma remains more controversial, and few surgeons advocate it in the setting of invasive melanoma.10
 Evaluating Metastatic Disease
Evaluating Metastatic Disease
Involvement of the major salivary glands can occur by direct invasion, lymphatic metastasis from a nonsalivary gland primary, and hematogenous spread from a distant primary. Direct invasion of the parotid gland is expedited by an interconnected plexus of periglandular and intraglandular lymph nodes. Submandibular lymph nodes lie outside the capsule of the gland, and parenchymal involvement would be by direct extension from these lymph nodes. Greater direct sun exposure to the preauricular region in comparison to the submandibular region leads to more direct invasion of the parotid gland by skin cancer (see Chapter 2, Fig. 2–1).
Ten to 15% of malignant neoplasms in the major salivary glands are from cancer metastases. Higher rates occur in Australia, where an elevated incidence of cutaneous malignancy pervades. Head and neck cutaneous squamous cell carcinoma and malignant melanoma make up 80 to 85% of metastatic tumors to the major salivary glands.3 Cutaneous squamous cell carcinoma with metastasis to the parotid is more common than melanoma. Its spread is via the lymphatic system. Ninety percent of such carcinoma is to the parotid. The balance is to the lymph nodes surrounding the submandibular gland. Metastasis to the sublingual gland has not yet been reported. The nasopharynx is the most common upper aerodigestive primary site that metastasizes to the parotid.
Metastatic disease to the salivary glands occurs later in life, with peak incidences in the sixth and seventh decade of life, predominantly in males. In melanoma and infraclavicular metastasis to the salivary glands, the first manifestation of disease may be the salivary gland mass. Kidney, lung, and breast are the most common sites of distant metastasis to the salivary glands. Prostate, colon, pancreas, stomach, bladder, uterus, and ovary are other sites. Careful evaluation usually results in identification of widespread metastasis once the salivary glands are involved with infraclavicular metastasis. Local control with a solitary metastasis to the salivary gland is possible.
 Basal Cell Carcinoma
Basal Cell Carcinoma
Basal cell carcinoma involving the parotid is usually from direct extension. Fewer than 200 cases of metastatic basal cell carcinoma have been reported. Metastatic basal cell carcinoma tends to occur in a younger population than nonmetastatic basal cell carcinoma, with an average age of 45.11 The time period between diagnosis of the initial lesion and metastasis is on average 6 to 9 years, sharply contrasting to the early appearance of metastatic squamous cell carcinoma.12 The risk factors that have been identified include recurrent tumors, tumors in patients with a history of radiation therapy, tumors greater than 10 cm2, and tumors of the scalp and face. The rare basal cell carcinoma meeting all four criteria may have up to a 50% chance of metastasis. Metastatic basal cell carcinoma occurs most notably in the setting of the morphea-form subtype.13 Unfortunately, the limited number of reports of metastatic basal cell carcinoma does not offer a common approach to this rare entity. Up to two thirds of the cases appear to have lymphatic spread; therefore, sentinel node biopsy may have a future role in the evaluation of the high-risk patient.13 Regional metastases have been noted mostly in the parotid and periparotid nodes. When metastatic basal cell carcinoma to the parotid is present, a total parotidectomy with preservation of the facial nerve (when not grossly involved) and a selective neck dissection (for the otherwise N0 neck) are recommended. A careful search for distant metastasis should precede surgery. In the patient with comorbidities, radiation therapy should be considered. The prognosis for basal cell carcinoma metastatic to the parotid is poor, with 6-month and 1-year survival at 50% and 20%, respectively.12
 Cutaneous Squamous Cell Carcinoma
Cutaneous Squamous Cell Carcinoma
Five percent of cutaneous squamous cell carcinoma patients have metastatic disease to the parotid or neck, occurring usually within 1 year of the index cancer.14 Metastatic disease to the parotid or neck may potentially not present for 2 or 3 years after resection of the primary lesion. These must be distinguished from the more unusual squamous cell carcinoma from a distant primary site and a primary parotid squamous cell carcinoma (a diagnosis of exclusion). Histological evaluation will not distinguish between these entities.15
The following risk factors are associated with metastases: tumor diameter greater than 2 cm, thickness greater than 4 mm, local recurrence, poorly differentiated tumors, rapid growth, perineural or perivascular invasion, location on the preauricular skin, external ear (with cartilage invasion), lip (with diameter greater than 1.5 cm), upper lip, within a scar or a non-sun-exposed area, and in patients who are immunocompomised.14 The occurrence of metastatic disease from cutaneous squamous cell carcinoma to the parotid gland correlates with the local control rate. The majority of parotid metastases occur within 1 year of treatment of the primary tumor and often occur simultaneously with local recurrence.16 Preauricular lesions have a high rate of recurrence and have the highest propensity for secondary involvement of the parotid (10% rate). Superficial parotidectomy should be considered in the primary treatment in selected preauricular skin squamous cell carcinomas. External ear and periauricular carcinoma have a propensity to invade the parotid, postauricular, and/or upper jugular nodes. Additional associated adverse prognostic factors would warrant strong consideration for elective parotidectomy with supraomohyoid and postauricular dissection for external ear and periauricular squamous cell carcinoma.17 In patients who developed external ear squamous cell carcinoma and regional metastases, 100% had parotid involvement, and 57% had cervical nodal spread.18
Lesions on the lip are associated with higher metastatic potential than other cutaneous sites.19 The lateral lip drains to the submandibular nodes. The middle third of the lip drains to the submental nodes, either submandibular nodes or bilateral submandibular nodes. Upper lip cancers drain primarily to the submandibular nodes, but also to the preauricular, parotid, and submental nodes. A squamous cell carcinoma of the lip must be distinguished from mucoepidermoid carcinoma of a minor salivary gland origin. Poorly differentiated mucoepidermoid carcinomas will contain a relatively high number of squamous cells,20 and a biopsy may only reveal this component to the pathologist. The high-grade mucoepidermoid carcinoma is an even more aggressive tumor with a greater metastatic potential.
Periparotid lymph node involvement carries a better prognosis than parenchymal metastatic parotid disease. Parotid parenchymal metastasis from a cutaneous squamous cell carcinoma primary has a reported 50% rate of recurrence and 20% mortality rate despite aggressive surgery and postoperative radiation therapy. Facial nerve weakness has been reported on initial presentation in 30% of cases, requiring nerve sacrifice in close to half of all cases.2
Metastatic cutaneous squamous cell carcinoma to the parotid is associated with a high incidence of clinical and occult neck metastases. A 25% rate of clinical neck metastasis can be expected in a patient with a parotid metastasis from a cutaneous primary. A 35% rate of positive neck lymph nodes with selective neck dissection has been reported in patients with parotid metastasis from a cutaneous primary and an N0 neck.21 The N0 neck with a metastasis to the parotid from a skin cutaneous primary from the anterior scalp, face, ear, and anterior neck should be treated with an anterolateral selective neck dissection (levels I, II, III, and IV) or radiation therapy. Posterior lesions would be treated with a posterolateral selective neck dissection (levels I, II, III, and V) or radiation.22
As the parotid gland develops, 20 to 30 lymph nodes are encased within it23 (accounting for the fact that the parotid is the salivary gland most involved with metastatic disease). Pathologic and clinical evidence suggests between one and five deep parotid lymph nodes exist in 90% of patients.24 Computed tomography (CT) or magnetic resonance imaging (MRI) may help determine if deep parotid or parapharyngeal nodes are present. In the presence of palpable intraparotid metastasis, a total parotidectomy with preservation of the facial nerve, when not grossly involved with tumor, offers the patient an anatomically appropriate operation.24,25 However, many surgeons favor a lateral lobectomy without clinical evidence of deep lobe involvement. Tumor control has not been proven higher with total parotidectomy or sacrifice of the facial nerve when the nerve was not grossly involved.1 Local recurrence in the parotid bed is the most common site of failure in cutaneous squamous cell carcinoma metastatic to the parotid.2 A 75% rate of recurrence with surgery alone16 has led to combined modality treatment with radiation therapy. Dona et al26 reported an improved 24% locoregional recurrence rate after parotidectomy and postoperative radiation, with recurrence occurring in a median time of less than 8 months. Radiation therapy should include the deep lobe if not resected (see Chapter 13).
Metastatic disease to the parotid carries a significant adverse clinical prognosis. The size of the parotid metastasis (<3 cm vs >3 cm) carries a significant adverse clinical prognosis. Metastatic cutaneous squamous cell carcinoma in both the parotid gland and cervical lymph nodes carries a worse prognosis than those with disease in the parotid alone. A TNM staging that separates parotid and neck stage may be valid.27
 Management of the Parotid Gland in the Presence of Cervical Metastatic Disease
Management of the Parotid Gland in the Presence of Cervical Metastatic Disease
Skin cancer patients may present with clinically positive neck disease without obvious parotid metastasis. In these individuals, the parotid nodes should be evaluated if they are within the path of spread. Cancers occurring lateral to the nasal sidewall and anterior to the coronal plane of the external auditory canal place the parotid in the path of spread. Most clinicians favor lateral lobectomy in the absence of palpable intraparotid nodes. In those patient, postoperative radiation therapy should include the remaining portion of the parotid gland. Neck metastases from skin cancers occurring posterior to the coronal plane of the external auditory canal do not warrant a parotidectomy.
Melanoma
The majority of melanomas involving the parotid gland result from metastasis from a head and neck cutaneous primary. Melanocytes within the parotid gland acinar and ductal cells support the remote possibility of primary melanoma of the parotid.28 To support a diagnosis of primary melanoma, the tumor epicenter should be in the parotid with no lymph node tissue in the mass. Unknown primary is a more likely scenario than primary melanoma of the parotid. Primary melanomas are known to occasionally undergo regression. Patients with unknown primary melanomas may have a survival advantage over patients with known primary.29
In patients with cutaneous malignant melanoma (CMM) of the head and neck, regional metastatic rates correlate with tumor thickness. Occult regional metastasis is 5% in tumors less than 1 mm, 20% for tumors between 1 and 4 mm, and up to 50% for tumors greater than 4 mm. However, no prospective trial has shown a survival advantage with elective lymphadenectomy partly because only 10 to 20% of patients present with occult nodal disease. Sentinel node biopsy (SNB) may spare a more radical procedure in most patients. SNB provides prognostic information and identifies patients who need lymphadenectomy and may benefit from adjuvant therapy or who may qualify for clinical trials. It is appropriate for T2N0M0, T3N0M0, and T4N0M0 CMM lesions.
Head and neck lymphatic drainage from the skin has unexpected drainage patterns with occasional bilateral or contralateral drainage. Skin metastasis to the neck without parotid involvement has been reported in 36% of patients.30 Most large series show a 95% success rate in localizing the sentinel node when preoperative lymphoscintigraphy and a handheld gamma probe are combined with blue dye injection.31,32 The two techniques are complementary because the sentinel lymph node containing micrometastasis may stain blue but not be radioactive or be radioactive and not stain blue. A sentinel lymph node found to be positive for micrometastasis is frequently the only node with metastatic disease.
Lymphoscintigraphy using technetium Tc 99m sulfur colloid has a low exposure to radiation for patients and physicians. For CMM, 0.5 mCi Tc 99m-labeled sulfur colloid is injected intradermally around the primary tumor. Imaging can occur 20 minutes after injection to determine likely sentinel nodes. The patient is taken to the operating room. Before skin incision, the gamma probe can identify a sentinel node within 1 cm, and the skin is marked in this location. The gamma probe can visualize a lymph node smaller than 5 mm. The gamma probe must be pointed away from the primary lesion because the primary lesion causes high background noise. Because the probe is held against the tissue during dissection, nodes in close proximity can be distinguished. Nodes that are more than 10% as hot as the hottest node are removed. Isosulfan blue dye, 1.0 mL, is injected intradermally around the primary tumor in the operating room prior to skin incision. Extravasation of dye staining the surgical field and obscuring tissue planes is a risk.
SNB reduces the number of nodes harvested and allows meticulous testing on the most important nodes. This cannot be done at frozen section. In addition to hemaoxylin and eosin staining, immunohistochemistry staining with HMB-45 and S-100 is performed. Reverse transcriptase polymerase chain reaction technology is also utilized. A differentiation must be made between melanoma and poorly differentiated carcinoma, adenocarcinoma, and anaplastic carcinoma. Desmoplastic melanoma, a spindle-shaped tumor mimicking other spindle cell tumors, including malignant myoepithelioma, sarcomatoid carcinoma, and malignant schwannoma, involves the parotid by neurotropic spread.33
Sentinel node biopsies are in their infancy, and the long-term utility will come to light when patients with negative sentinel nodes are followed over many years. To date there is no evidence of improved survival with SNB for cutaneous malignant melanoma.
Thirty-eight percent of patients with parotid metastasis from a CMM have been reported to have clinical cervical lymph node metastasis. In addition, 27% of patients with parotid metastasis from CMM will have occult cervical neck metastasis in the N0 neck.21 Patients with parotid metastasis and N0 neck should be considered for elective neck dissection. Radiotherapy improves regional control but not overall survival. Metastatic melanoma to the parotid carries a grim prognosis with a high rate of distant metastasis.
Surgical Technique in Sentinel Lymph Node Biopsy of the Parotid Gland
Up to 50% of head and neck melanomas drain to the periparotid region. Many reports describe SNB in the parotid without identification of the facial nerve. As the utility of and familiarity with SNB increases among surgeons, this trend of “berry picking” may continue. The incidence of iatrogenic facial nerve paralysis will climb if that method gains a foothold. Reports that describe a lack of facial nerve dissection come in two varieties: Either the authors fail to appreciate the danger to the facial nerve, or the authors do appreciate the risk but feel that blunt dissection will spare the nerve.34,35
Table 11-1 TNM Classification for Malignant Melanoma
| pTNM Pathological classification |
| pT1a 1 mm or less in thickness, no ulceration |
| pT1b 1 mm or less in thickness, with ulceration |
| pT2a>1–2 mm, no ulceration |
| pT2b>1–2 mm, ulceration |
| pT3a>2–4 mm, no ulceration |
| pT3b>2–4 mm, with ulceration |
| pT4a>4 mm, no ulceration |
| pT4b>4 mm, with ulceration |
| N1a 1 node, microscopic |
| N1b 1 node, macroscopic |
| N2a 2–3 nodes microscopic |
| N2b 2–3 nodes macroscopic |
| N2c satellite or in transit without nodes |
| N3 4 or more nodes; matted |
| Stage grouping |
| Stage I and II: N0 |
| Stage III: Any pT, N1, N2, N3 |
| Stage IV: Any pT, any N, M1 |
pT, primary tumor
Blunt dissection through the parotid gland in search of a potential malignancy risks not only the nerve during that procedure. If an intraparotid sentinel node contains cancer, then the nerve will need a formal dissection anyway to remove the gland. If the surgeon has not done a dissection to find the node and finds cancer in that node, then the subsequent nerve dissection is inevitably more treacherous. The TNM staging system for melanoma is shown in Table 11-1.36
REFERENCES
1. Khurana, VG Mentis, DH O’Brien, CJ, et al. Parotid and neck metastases from cutaneous squamous cell carcinoma of the head and neck. Am J Surg 1995; 170: 446–450
2. Lai, SY Weinstein, GS Chalian, AA, et al. Parotidectomy in the treatment of aggressive cutaneous malignancies. Arch Otolaryngol Head Neck Surg 2002; 128: 521–526
3. Batsakis, JG Bautina, E. Metastases to major salivary glands. Ann Otol Rhinol Laryngol 1990; 99: 501–503
4. Schwartz, RA Stoll, HL. Squamous cell carcinoma. In: Fitzpatrick, TB Eisen, AZ Wolff, K Freedberg, IM Austen, KF, eds. Dermatology in General Medicine. New York: McGraw-Hill; 1993: 821–839
5. Levi, F LaVeccia, C Te, VC Randibison, L Erler, G. Incidence of invasive cancers following basal cell skin cancer. Am J Epidemiol 1998; 147 (8): 722–726
6. Levi, F Randimbison, L La Vecchia, C Erler, G Te, VC. Incidence of invasive cancers following squamous cell skin cancer. Am J Epidemiol 1997; 146 (9): 734–739
7. Kahn, HS Tatham, LM Patel, AV, et al. Increased cancer mortality following a history of nonmelanoma skin cancer. JAMA 1998; 280 (10): 910–912
8. Milan, T Pukkala, E Verkasalo, PK et al. Subsequent primary cancers after basal-cell carcinoma: a nationwide study in Finland from 1953 to 1995. Int J Cancer 2000; 87: 283–288
9. Cook, J Zitelli, JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol 1998; 39 (5, pt 1): 698–703
10. Bricca, GM Brodland, DG Ren, D Zitelli, JA. Cutaneous head and neck melanoma treated with Mohs micrographic surgery. J Am Acad Dermatol 2005; 52 (1): 92–100
11. von Domarus, H Stevens, PJ. Metastatic basal cell carcinoma. J Am Acad Dermatol 1984; 10: 1043–1056
12. Lo, JS Snow, SN Reizner, GT, et al. Metastatic basal cell carcinoma: report of twelve cases with a review of the literature. J Am Acad Dermatol 1991; 24: 715–719
13. Malone, JP Fedok, FG Belchis, DA, et al. Basal cell carcinoma metastatic to the parotid: report of a new case and review of the literature. Ear Nose Throat J 2000; 79 (7): 511–515
14. Rowe, DE Carroll, RJ Day, CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip: implications for treatment modality selection. J Am Acad Dermatol 1992; 26: 976–990
15. Taxy, J. Squamous carcinoma in a major salivary gland. Arch Pathol Lab Med 2001; 125: 740–745
16. Cassisi, NF Million, RR. Management of head and neck cancer: a multidisciplinary approach. Philadelphia: JB Lippincott; 1994
17. Byers, R Kesler, K Redmon, B, et al. Squamous carcinoma of the external ear. Am J Surg 1983; 146: 447–450
18. Lee, D Nash, M Har-El, G. Regional spread of auricular and periauricular cutaneous malignancies. Laryngoscope 1996; 106: 998–1001
19. Dinehart, SM Pollack, SV. Metastases from squamous cell carcinoma of the skin and lip: an analysis of twenty-seven cases. J Am Acad Dermatol 1989; 21: 241–248
20. Cotran, RS Kumar, V Robbins, SL, et al. Diseases of the head and neck. In: Robbins, SL, ed. Robbins’ Pathologic Basis of Disease. Philadelphia: WB Saunders; 1989: 811–826
21. O’Brien, CJ McNeil, EB McMahan, JD Pathak, I Louer, CS. Incidence of cervical node involvement in metastatic cutaneous malignancy involving the parotid gland. Head Neck 2001; 23: 744–748
22. Shah, JP Kraus, DH Dubner, S, et al. Patterns of regional lymph node metastasis from cutaneous melanoma of the head and neck. Am J Surg 1991; 162: 320–323
23. Conley, J. The significance of the parotid gland as a focus of metastasis. In: Conley, J, ed. Salivary Glands and the Facial Nerve. Stuttgart, Germany: Georg Thieme Publishers; 1975
24. Pisani, P Ramponi, A Pia, F. The deep parotid lymph nodes: an anatomical and oncological study. J Laryngol Otol 1996; 110: 148–150
25. Bron, LP Traynor, S McNeil, E O’Brien, CJ. Primary and metastatic cancer or the parotid: comparison of clinical behavior in 232 cases. Laryngoscope 2003; 113: 1070–1075
26. Dona, E Veness, MJ Cakir, B Morgan, GJ. Metatstatic cutaneous squamous cell carcinoma to the parotid: the role of surgery and adjuvant radiotherapy to achieve best outcome. ANZ J Surg 2003; 73: 692–696
27. O’Brien, C McNeil, E McMahon, J Pathak, I Lauer, C Jackson, M. Significance of clinical stage, extent of surgery, and pathologic findings in metastatic cutaneous squamous carcinoma of the parotid gland. Head Neck 2002; 24 (5): 417–422
28. Prayson, R Sebek, B. Parotid gland malignant melanomas. Arch Pathol Lab Med 2000; 124: 1780–1784
29. Wang, B Lawson, W Robinson, R Perez-Ordonez, B Brandwein, M. Malignant melanomas of the parotid. Arch Otolaryngol Head Neck Surg 1999; 125: 635–639
30. Martins, AS Souza, AL Souza, LS Lage, HT. Surgical procedures for primary, metastatic or adjacent parotid tumours. Int Surg 1999; 84: 318–325
31. Patel, SG Coit, DG Shaha, AR, et al. Sentinel lymph node biopsy for cutaneous head and neck melanomas. Arch Otolaryngol Head Neck Surg 2002; 128 (3): 285–291
32. Thomas, JM Patocskai, EJ. The argument against sentinel node biopsy for malignant melanoma. BMJ 2000; 321 (7252): 3–4
33. Jennings, TA Okby, NT Schroer, KR Wolf, BCV Mihm, MC. Parotid involvement by desmoplastic melanoma. Histopathology 1996; 29: 165–170
34. Weisberg, NK Bertagnolli, MM Becker, DS. Combined sentinel lymphadenectomy and Mohs micrographic surgery for high risk cutaneous squamous cell carcinoma. J Am Acad Dermatol 2000; 43 (3): 483–488
35. Ollila, DW Foshag, LJ Essner, R Stern, SL Morton, DL. Parotid region lymphatic mapping and sentinel lymphadenectomy for cutaneous melanoma. Ann Surg Oncol 1999; 6 (2): 150–154
36. Sobin, LH Wittekind, C. TNM Classification of Malignant Tumours. West Sussex, England: John Wiley & Sons; 2002
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


