
10

Malignant Neoplasms of the Salivary Glands
Malignant salivary gland tumors are uncommon and account for less than 1% of all malignancies and ~ 5% of head and neck cancers. There is an annual incidence of 1 to 2 per 100,000 worldwide.1,2 These tumors comprise a wide spectrum of phenotypic and biological entities. Given their rarity and biological diversity, there is little evidence-based data to provide conclusive recommendations for treatment and outcome measures for these patients. This is compounded by the development of these tumors in a wide variety of anatomical sites, including both minor and major salivary glands. Unique among head and neck tumors, those of salivary origin may aptly fit the admonition of Hippocrates: “Experience fallible, judgment difficult.” Despite these difficulties, general guidelines for the management of these tumors can be drawn from the literature and are presented herein.
 Overview
Overview
Epidemiology
The Surveillance, Epidemiology, and End Results (SEER) and American Cancer Society registries record salivary gland tumors (benign and malignant) in the anatomical site of origin (e. g., oral cavity and pharynx), rather than within a unique, organ-specific salivary gland category. This method of epidemiologic tabulation makes it difficult to determine the precise incidence of this rare disease. Nonetheless, a population-based study suggests the annual age-adjusted incidence for salivary malignancy is 0.9 per 100,000 (in US).3
Most salivary tumors (80%) arise in the parotid gland, and the majority of these (65%) are benign. Tumors of the submandibular gland account for 10 to 15%, with a higher incidence of malignancy, up to 50%. The remaining 5 to 10% arise in minor salivary glands, but almost 80% of these tumors are malignant. Very rarely, salivary gland malignancy may develop in heterotopic salivary tissue, primarily the lymph nodes and bones of the head and neck. For these heterotopic tumors, an adjacent or related primary tumor must be ruled out with careful examination and imaging.
There is no clear causative relationship between the development of salivary gland tumors and tobacco or ethanol use. However, ultraviolet radiation exposure has been suggested to play an etiologic role.4–6 A review of the SEER epidemiological database from 1973 to 1981 demonstrated significantly higher rates of salivary malignancy for white males and females in the southern United States compared with the North, for all histological subtypes combined.5
Exposure to gamma radiation has also been suggested to play a role in salivary gland tumorigenesis. The rates of salivary malignancy among survivors of atomic bomb blasts and in patients treated with head and neck radiation for benign conditions are increased compared with controls.7,8 Zheng et al have suggested a possible molecular mechanism for the putative association between gamma radiation and the development of salivary gland tumors.9 Dental x-rays, nickel alloys, silica dust, kerosene, industrial rubber exposure, and hair dyes might have an association.
Embryology and Histogenesis of Salivary Gland Tumors
Salivary glands are ectodermally derived and develop from proliferations of oral epithelium that grow and invaginate into the underlying mesenchymal stroma. These solid, epithelial ingrowths canalize and then branch into tubules, which differentiate into the mature salivary gland structure (Fig. 10–1).
The functional unit of all major salivary gland tissue is the acinus. It is composed of mucinous, serous, and seromucinous cells that are surrounded by myoepithelial cells. Acini are linked to the intercalated ducts, then into mitochondria-rich striated ducts, and finally into the excretory ducts. The cellular composition varies according to the location and type of salivary gland. In the parotid, serous cells predominate, whereas the sublingual gland is mainly composed of mucinous cells. Submandibular glands are composed of both mucinous and serous elements.
The parotid gland, from an embryologic standpoint, develops early and represents the most complex structure, with the most pure acinus formation. The intercalated and striated ducts lead to excretory ducts that empty in Stensen’s duct. The minor salivary glands lack a ductal network, and each secretory unit drains individually and varies in its cellular composition based on its location.
The histogenesis of salivary gland neoplasms has been the subject of great debate. The bicellular stem cell or reserve cell theory10 proposed that salivary neoplasms are derived from unique stem cells with the capacity for self-renewal. In this hypothesis, two types of basal reserve cells (or stem cells) from the intercalated and excretory ducts provide the origin for most epithelial salivary neoplasms. Accordingly, epidermoid tumors, such as squamous cell carcinoma and mucoepidermoid carcinoma, are thought to arise from excretory duct reserve cells, whereas glandular tumors, such as adenocarcinomas, adenoid cystic carcinomas, and acinic cell carcinomas, arise from intercalated duct reserve cells.
An alternative hypothesis has recently been proposed. Known as the multicellular theory,11 all cell types in the salivary gland unit (10–1) are capable of replication and, therefore, of being involved in tumorigenic processes. Thus myoepithelial cells give rise to a remarkably heterogeneous range of tumors, includingacinic cell carcinoma and adenoid cystic carcinoma. Terminal duct (also known as polymorphous low-grade) adenocarcinoma develops from the terminal interca lated duct and acini.12,13 Tumors with oncocytic features because of increased cytoplasmic mitochondria may arise from striated ductal cells. Mucoepidermoid, salivary duct, and, rarely, primary squamous carcinomas arise from the excretory duct.
General Considerations
A slow-growing, painless mass is the presenting feature of most benign and malignant salivary gland neoplasm. This commonality may lead to a delay in diagnosis of malignant salivary gland tumors. The minority of tumors present with pain that is usually continuous, rather than intermittent. Facial nerve dysfunction, adenopathy, trismus, numbness, fixation, loose dentition, and bleeding are strongly suggestive of malignancy. Imaging, in general, will not distinguish a malignant from benign neoplasm. Sialography has no significant role. Ultrasound may play a role in fine-needle aspiration of a neoplasm with solid and cystic features. Computed tomography (CT) and magnetic resonance imaging (MRI) will define the extent of tumor and are most important for large, deep lobe, and parapharyngeal space lesions (see Chapter 2, Figs. 2–3, 2–4, 2–5, 2–13, 2–14, 2–15). CT has the advantage of defining cortical bone involvement, whereas MRI provides superior soft tissue detail. Both provide surveillance of the neck. False-positive diagnosis of malignancy by fine-needle aspiration and frozen section can occur; these studies should not dictate sacrifice of the facial nerve.
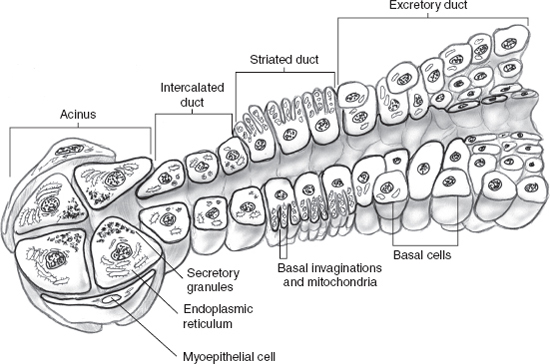
FIGURE 10-1 Salivary gland unit.
Treatment of the Primary Tumor
Surgery is the primary treatment for malignant salivary gland tumors. The guiding surgical principal is wide excision of the tumor with an adequate margin of surrounding salivary gland or other tissue. However, different surgical approaches are generally indicated based on whether the neoplasm arises in the major (parotid, submandibular, or sublingual) or minor salivary glands.
The Parotid Gland
Because most parotid tumors arise in the superficial lobe, a superficial parotidectomy with a wide cuff of gland generally provides an adequate excision. Total parotidectomy is not indicated for all malignant parotid gland neoplasms unless the tumor arises within the deep lobe or there is direct extension from the superficial to deep lobe.
If the facial nerve functions preoperatively, every effort should be made to preserve it. Facial nerve dissection and preservation may leave disease at the tumor nerve interface, but the nerve should not be sacrificed for microscopic control of tumor. Postoperative radiation therapy is often effective in this situation for controlling microscopic disease in the parotid bed. Occasionally, in advanced tumors, resection of adjacent structures is indicated when there is intraoperative evidence of invasion. In this setting, the nerve may require resection. If necessary, frozen section margins are obtained from the proximal and distal nerve endings.
The Submandibular Gland
Preoperative fine-needle aspiration biopsy is mandatory for surgical treatment of submandibular lesions. Because the surgical approach differs significantly for inflammatory versus neoplastic lesions, the fine-needle aspiration result can prepare both the surgeon and patient for the anticipated surgical resection. However, intraoperative frozen section confirmation must ultimately guide the final surgical approach because extensive surgery should not be planned on the basis of fine-needle aspiration results alone.
If malignancy is known or suspected, a limited excision of the gland alone should be avoided. Therefore, a wide excision with planned level Ia–Ib neck dissection is necessary to obtain an appropriate soft tissue margin and to remove the primary echelon lymphatics. The hypoglossal and lingual nerves should always be preserved unless directly invaded or if their function is abnormal preoperatively. During a planned submandibular resection for malignancy, sampling of both the submandibular ganglion and the nerve to mylohyoid should be routinely performed to determine the presence of perineural invasion.
The Sublingual Gland
Tumors of the sublingual gland are quite rare, but they should always be considered during the evaluation of a submucosal anterolateral floor of the mouth lesion. Fine-needle aspiration biopsy is difficult, and an initial limited incisional biopsy is therefore indicated. Once confirmed, the surgical approach should encompass a wide resection, with a margin of overlying mucosa and a formal floor of the mouth resection. Preoperative counseling must include a discussion of the needed reconstruction and the possible need for temporary tracheotomy. Sampling of the adjacent nerve branches should be performed to determine perineural involvement, but again the lingual and hypoglossal nerves should be preserved unless directly involved or encased.
Minor Salivary Glands
Because of the diverse distribution of minor salivary glands within the head and neck, designing treatment can be difficult. There may be between 500 and 750 minor salivary glands within the submucosa of the upper aerodigestive tract. For optimal local and regional control, surgical resection is recommended. Although it is tempting to consider alternative treatments because of the associated morbidity of surgical resection, especially in the base of tongue (BOT) and larynx, disease control is often compromised.
Because the minor salivary glands are found in the highest density within the oral cavity, in particular the hard palate, minor salivary malignancy presents here most often. Fortunately, resection and functional reconstruction with minimal morbidity are often possible for patients with these tumors. In the oropharynx, wide resection and reconstruction are often most appropriate, but postoperative radiotherapy is often required. For the larynx, tumors commonly arise in the supraglottic and subglottic larynx. When appropriate, a conservation approach should be considered, but in practice indications are rare.
The Neck
Neck dissection should be performed when there is clinical or radiographic evidence of regional metastasis. Although some have advocated routine prophylactic lymphadenectomy, there are no evidence-based data to support this approach, especially for patients who would receive radiotherapy for perineural invasion. However, when the tumor arises close to the primary draining nodal echelons in an N0 neck, a selective, function-sparing lymphadenectomy (zones 1, 2, and 3) can be performed to guide postoperative therapy. For instance, it is our practice to perform an elective neck dissection in patients with high-grade mucoepidermoid carcinoma (MEC) of the parotid and submandibular glands because there is a well-documented increased incidence of occult positive nodes.14 In general, unless there are compelling pre-or intraoperative indications, neck dissection should not be routinely performed. Tumors at risk for occult metastasis include high-grade tumors (squamous cell carcinoma, high-grade adenocarcinoma, high-grade mucoepidermoid carcinoma, and undifferentiated carcinoma), size greater than 4 cm, tumors presenting with facial nerve dysfunction, extraglandular involvement, advanced age, recurrent tumors, and submandibular tumors.
Surgical Management of Cranial Nerves
Cranial Nerve VII
Occasionally, salivary tumors must be sharply dissected from the facial nerve, leaving microscopic disease behind. This should only be done when no gross tumor is left behind. For these patients, postoperative radiotherapy is necessary.
If the facial nerve functioned preoperatively but was sacrificed during surgery, nerve grafting should be performed. Reconstruction can be achieved with either direct neurorrhaphy (if the defect is small and the nerve edges mobile) or an interposition nerve graft or cable graft, depending on the length of the resected segment.
If the facial nerve was paralyzed preoperatively, reanimation of the face should be considered. For curative resections, a temporalis muscle transfer, fascial sling, or similar technique is appropriate. Because postoperative radiation is necessary, any reanimation approach utilizing foreign material or implants must be avoided. Gold weight implantation into the upper eyelid also should be considered in either case.
A mastoidectomy is occasionally necessary to achieve negative margins on the proximal stump of the facial nerve. When the main trunk of the facial nerve is sacrificed at the stylomastoid foramen, a mastoidectomy provides access and nerve mobility to perform an interposition nerve graft. Finally, in the setting of extensive scarring from previous surgery or with recurrent disease, a mastoidectomy may facilitate the identification of the main trunk of the facial nerve.
For advanced salivary tumors extending beyond the confines of the parotid gland, preoperative imaging allows for better delineation of the extent of the resection of surrounding structures. Preoperative planning and postoperative reconstruction must consider the need to resect skin, mandibular ramus, and masseter muscle; infratemporal fossa dissection; or subtotal petrosectomy. A lateral temporal bone resection is indicated when the tumor involves the external auditory canal or the temporomandibular joint.
Cranial Nerve V
If the lingual nerve is involved by submandibular or sublingual tumors, all gross tumor should be resected and the nerve dissected toward its ganglion. A microscopically negative margin should be obtained when possible. If multiple margins remain positive to the foramen ovale, the nerve stump should be marked with a metal clip, to assist in planning the radiation field.
For minor salivary gland tumors arising in the hard palate, infrastructure maxillectomy with split-thickness skin graft and prosthetic reconstruction is often necessary. For these patients, resection of the inferior turbinate facilitates obturation. Branches of the maxillary division of the trigeminal nerve (infraorbital and greater palatine nerves) should be biopsied if perineural invasion is suspected. For larger tumors requiring total maxillectomy, the branches of the second (V2) and third (V3) divisions of the trigeminal nerve are at high risk for perineural spread and should be sampled. Although these nerves may provide an avenue for early skull base and intracranial disease, a balanced approach must be selected because, regardless of the extent of surgery, postoperative radiation therapy will be necessary.
Adjuvant Radiation Therapy
Postoperative Radiation Therapy
Indications for postoperative x-ray therapy are listed in Table 10-1 (see Chapter 13 for an expanded discussion). The typical recommended dose is ~ 60 Gy. Treatment volumes depend on the tumor histology, location of the primary tumor, and the risk of tumor involvement in the neck and to neural pathways.
Adjuvant postoperative x-ray therapy leads to improved local control especially for T3–4 and highgrade tumors. 15–18 In an older series, postoperative radiotherapy has been shown to reduce local recurrence from ~30 to 10%.19 Skip metastases may occur in levels III and IV with negative level II nodes in up to 25% of patients undergoing elective neck dissections.20
Table 10-1 Indications for Postoperative Radiotherapy
| 1. Compromised margins of resection, including tumor enucleation |
| 2. Extraglandular extension |
| 3. Facial nerve preservation with close margins (tumor peeled away) |
| 4. Perineural invasion |
| 5. Metastatic lymphadenopathy |
| 6. High-grade tumors |
| 7. Recurrent low-grade tumors with no good salvage option on future recurrence |
It is hard to demonstrate an objective improvement in survival by any modality of adjuvant radiation therapy; however, a matched-pair retrospective analysis at Memorial Sloan Kettering Cancer Center suggested improved local control and survival for adjuvant radiotherapy for all patients with positive nodes and stage III and IV disease.20 The salvage rates are nil for many patients who subsequently develop recurrences.
Data from the MD Anderson Cancer Center indicate excellent locoregional control and modest complication rates for patients with parotid cancers treated with ipsilateral electron fields directed at the parotid bed.17 The electron beam was often mixed with a small proportion of photons to improve skin sparing. Alternative treatment techniques evaluated included the ipsilateral “wedge-pair” technique using photons. The electron-based technique was appropriate for all but deeply seated tumors, and overall had a lower complication rate as compared with the wedge-pair technique. The locoregional control rate was 84%, and there was a 22% risk for chronic complications (hearing loss 7%, soft tissue necrosis 9%, temporal lobe necrosis 1%, or soft tissue/bone exposure/necrosis 9%).
Postoperative radiation therapy also using ipsilateral primarily electron or mixed beam technique for submandibular gland cancer has led to local control in~90% of patients.18 There was no salvage for patients with locoregional recurrence, underscoring the utility for postoperative radiation therapy. In this series of 94 patients, failure along nerves was rare. The authors recommend treatment of the nerve pathway to the skull base foramen only for patients with involvement of a named nerve, but covering the nerve’s pathway through the mandible and neck for patients with focal and microscopic perineural invasion. This limits the morbidity of unnecessarily large radiation therapy fields. Thus the risk for serious complications was lower than with the larger fields used for parotid cancers that also cover more complex and costly areas of anatomical function. There was a 5% incidence of osteoradionecrosis of the mandible.
Low-grade mucoepidermoid carcinomas have near uniform control with complete surgical excision. If there is compromised resection without a good option for reoperation to achieve that complete resection, or if observation and salvage are not a good option, then the results are improved with postoperative radiotherapy.19,21
Previous surgical series have reported local recurrence rates approximating 50%. This is not surprising because many patients have disease originating in paranasal sinus and palate sites. Postoperative radiation therapy has led to local control rates of 88%, and overall survival rates of 81%, 65%, and 43% at 5, 10, and 15 years, respectively.16 This decrement in survival reflects the preponderance (71%) of patients with adenoid cystic carcinomas, for whom the dominant pattern of failure was distant metastases. Failures in the neck were rare (<5%), so the authors recommend neck treatment only for the minority with nodal involvement, for tongue primaries, and for those whose neck was entered for resection of the primary, thus becoming a part of the operative bed. However, because of the unique natural history of adenoid cystic carcinoma and proclivity for perineural invasion, radiotherapy should be applied postoperatively. When involved, the facial nerve should be treated up to its point of entry into the base of the skull for parotid tumors.22,23 The microscopic involvement of unnamed nerves, however, does not require treatment to the skull base, but for a generous portion of the nerve path in the neck.
The involvement of one side of the neck does not place the other side at risk in salivary cancer, as is the case for many occurrences of squamous carcinoma of the head and neck. Two studies have shown that contralateral elective neck radiation is not indicated.24,25 This decreases the incidence/severity of xerostomia, and other potential risks and complications for the irradiation of unnecessary large volumes.
Gross Disease
There are data on the treatment of patients with gross residual salivary gland cancers following surgical resection. Conventional once-a-day photon radiation therapy has been associated with a local control rate of 20 to 25% for patients with inoperable or recurrent tumors (Table 10-2). Data on twice a day fractionation for photon radiation therapy are limited. One report, however, describes a 5-year actuarial local control of 100% for 9 parotid and 78% control for 15 minor salivary tumors.26 As twice-a-day radiotherapy has led to improved local control for other head and neck sites, it is reasonable to accept that it might lead to some improvement for salivary cancers, but the results of this trial have yet to be confirmed, and a more comprehensive evaluation of efficacy would require at least larger numbers (see Chapter 13).
Table 10-2 Local Control for Gross Tumor with Low Linear Energy Transfer, Once Daily
| Series | Numbers of patients | Control rate (%) |
|---|---|---|
| Fitzpatrick | 50 | 12 |
| Vikram | 49 | 4 |
| Borthne | 35 | 23 |
| Rafla | 25 | 36 |
| Fu | 19 | 32 |
| Stewart | 19 | 47 |
| Ellis | 17 | 29 |
| Dobrowsky | 17 | 41 |
| Shidnia | 16 | 38 |
| Elkon | 13 | 15 |
| Ravasz | 12 | 25 |
| Rossman | 11 | 54 |
| Overall | 283 | 24 |
A prospective clinical trial comparing photon radiation therapy to neutrons was undertaken by radiation therapy oncology group/medical research council (RTOG/MRC) for inoperable or recurrent salivary cancers. This demonstrated a greater than threefold improvement in the local control rate and a greater than twofold survival advantage at 2 years favoring neutrons. There was early closure of this trial at interim analysis for ethical reasons.26a A retrospective review of 263 patients treated with fast neutron radiotherapy for gross disease revealed a 6-year locoregional control rate of 59%.27
Photon radiation therapy should be used definitively for those patients with gross disease who cannot undergo surgery and for whom neutron beam therapy is not available or appropriate. In those circumstances, accelerated radiotherapy should be considered. The use of neutrons is not presently indicated in the setting of postoperative radiation therapy without gross disease.
Palliative Treatment
Neutron radiation may be a treatment option for inoperable locoregional disease or patients with comorbidities that preclude surgery. There are only two centers in the United States that can deliver this therapy, but this approach has been more widely used and studied in Europe.28 In 1988, a multicenter randomized study of 25 patients showed initially promising results in the locoregional control of unresectable salivary gland tumors, when neutron beam versus photon irradiation was compared. However, no survival difference could be demonstrated.26a,29
Chemotherapy
No single chemotherapeutic agent or combination regimen has shown any sustained efficacy for salivary tumors. Thus chemotherapy is indicated only for palliation of symptomatic patients with recurrent and/or unresectable cancers.30
Although unproven, it is widely supposed that there are differences in response to chemotherapy depending on the histogenic origin of the tumors.31 In general, tumors putatively arising from intercalated duct origin (adenoid cystic carcinoma, adenocarcinoma not otherwise specified, acinic cell carcinoma, terminal duct/polymorphous low-grade adenocarcinoma, and myoepithelial carcinoma) are low grade and biologically more indolent compared with those derived from the excretory duct, such as salivary duct, mucoepidermoid, and squamous carcinomas.
Accordingly, for tumors of intercalated duct origin, cyclophosphamide, doxorubicin, and cisplatin32,33 may offer the best response. Patients with mucoepidermoid, salivary duct, and undifferentiated carcinomas, however, appear to respond better to those drugs active against squamous cell carcinomas (e. g., cisplatin, 5-fluorouracil, and methotrexate). Unfortunately, for both groups, despite an occasional complete response, there has been no demonstrated survival benefit (see Chapter 12).
Molecular Targeted Therapy
HER2/neu (or c-erbB2) is a member of the epidermal growth factor receptor family. As a proto-oncogene, HER2 can dimerize with other c-ERB family members, and can initiate a cascade of downstream effects, leading to cell proliferation, angiogenesis, and resistance to programmed cell death, or apoptosis.34 In breast cancer, resistance to chemotherapy (and its attendant poorer prognosis) has been correlated with HER2 overexpression.35 Trastuzumab is a monoclonal antibody that selectively blocks the HER2 receptor and alone has single-agent activity with minimal side effects. This molecular targeted compound has shown promise in prospective clinical trials for patients with breast cancer, improving their response to standard cytotoxic therapy.36
In a multi-institutional study of patients with any histological type of salivary malignancy, HER2 overexpression was found in 10 of 12 (83%) patients with salivary duct carcinoma.31 For patients with salivary carcinoma, the vast majority of patients with HER2 overexpression had tumors arising from the excretory duct (Fig. 10–1): mucoepidermoid, squamous, and salivary duct carcinoma. Although these tumors represented a minority (22%) of the tumors in the study, there were striking responses in this group. In particular, two patients with salivary duct carcinoma had stable disease for 24 and 40 months. Unfortunately, with the small number of patients in the study, no conclusions could be drawn. But for patients with excretory duct-derived carcinomas, trastuzumab may provide hope for systemic therapy.
 Epithelial-derived Salivary Gland Malignancies
Epithelial-derived Salivary Gland Malignancies
Mucoepidermoid Carcinoma
Clinical Presentation
Mucoepidermoid carcinoma (MEC) is the most common malignant tumor of the salivary glands, making up more than one third of salivary malignant neoplasms. It is the most common malignant tumor of the parotid gland and the second most common tumor arising in the submandibular and sublingual glands. In children, MEC is the most common malignant tumor of salivary gland origin. The majority of patients present with a painless swelling of the area affected. Pain may occur, but it is usually preceded by the appearance of a mass. Cervical node involvement can be seen in one third of the patients. Primary lesions can be found mostly in the body and tail of the parotid gland, and presentation with facial palsy is not uncommon. High-grade MEC is associated with rapid growth at presentation. Minor salivary gland mucoepidermoid carcinoma presents most commonly in the buccal mucosa and palate.
Histology
The histological grading of mucoepidermoid carcinoma serves as a crucial prognostic factor and strongly correlates with clinical behavior. Its first histological description was made by Volkmann in 1895.37 Some 50 years later, Stewart et al coined the term mucoepidermoid carcinoma.38
Since then, a grading system has been developed based on the histological findings in which low-grade or well differentiated lesions show a better prognosis than39,40 high-grade or poorly differentiated tumors. The cardinal histological features include mucinous, intermediate, and epidermoid cellular elements, forming glandular, cystic, and solid arrangements.
Low-grade-type tumors show glandular or microcystic structures lined with a cell layer of mucus-producing cells (Fig. 10–2). The cystic structures can also have papillary infoldings with the presence of intermediate cells that may differentiate into epidermal and mucous cells. As some of these microcystic formations coalesce into larger cysts, some mucinous material may extrude and elicit an intense inflammatory reaction. Intermediate-grade tumors are characterized by areas of mostly squamous or intermediate basaloid-type elements (Fig. 10–3). High-grade forms show a greater percentage of solid sheets of tumor, with few glandular or cystic formations (Fig. 10–4). Intermediate basal and epidermoid cells are more predominant than mucus-producing cells in this aggressive form of MEC. Sclerosing mucoepidermoid carcinoma is characterized by an intense central sclerosis, frequently with dense collagenous depositions, ductal proliferations of mucinous and/or epidermoid cells, surrounded by plasma cells, eosinophils, or lymphocytes at the periphery.41,42
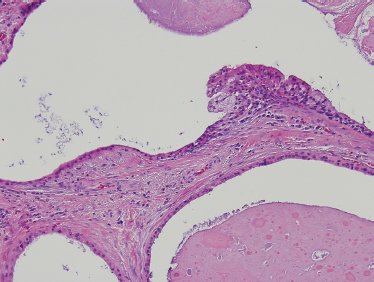
FIGURE 10-2 Low-grade mucoepidermoid carcinoma: largely cystic with small tumor nests (cystic) (H&E, ×20).
Histological grading of mucoepidermoid carcinomas of major salivary glands, using the modified Healey three-tiered system, correlates well with several important clinical prognostic features. In a study of 48 patients from the MD Anderson Cancer Center, the presence of lymphatic spread was closely correlated with increasing histological grade: 0% for low-, 22% for intermediate-, and 72% for high-grade tumors. (p <. 001). Furthermore, survival was decreased significantly (p <. 0001) with increasing tumor grade: 100% for low-, 70% for intermediate-, and 22% for high-grade tumors. Finally, perineural and lymphovascular invasion are not uncommon findings in MEC. Regional metastases can show cellular elements of all types not necessarily correlated to the primary lesion.
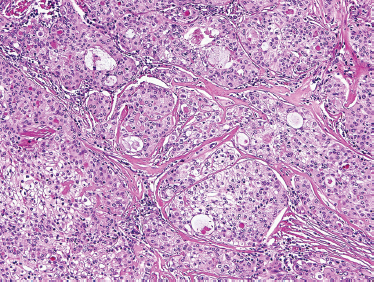
FIGURE 10-3 Intermediate grade mucoepidermoid carcinoma: predominately solid tumor nests with minor cystic formation (H&E,×40).
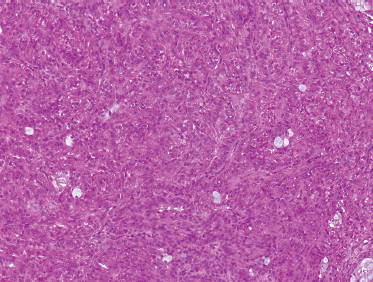
FIGURE 10-4 High-grade mucoepidermoid carcinoma: solid nests of poorly differentiated tumor cells (H&E,×20).
Treatment
Comprehensive surgical resection is the treatment of choice for MEC, depending on the location of the lesion. A complete superficial lobe parotidectomy with a wide cuff of gland around the tumor should provide an oncologically appropriate margin. Deep lobe parotidectomy should not be performed routinely but rather when tumor spread dictates. During resection of the primary, the facial nerve should be preserved whenever possible.
Neck dissection should be performed when there is clinical evidence of regional metastasis, either radiographically or clinically. Although some have advocated prophylactic lymphadenectomy for high-grade histology, there are no evidence-based data to support this approach, especially for patients who are scheduled to receive radiotherapy. Another relative indication for selective lymphadenectomy is the proximity of tumor to regional lymph nodes.
Postoperative radiotherapy is indicated in patients with perineural involvement, positive margins, high-grade tumors, and tumors with cervical lymphadenopathy.
Adenoid Cystic Carcinoma
Adenoid cystic carcinoma (ACC) is a relatively rare and indolently malignant salivary gland tumor with predilection for perineural invasion. It is well known, despite its infrequency, for the perplexing dilemmas it poses to patients and their treating head and neck oncologists.
The first recognition of ACC probably dates back to 185343 and 1854.44 Robin and colleagues first described these tumeurs hétéradéniques43,44. Two years later, writing in a monograph from the University of Berlin, Billroth coined the term cylindroma to describe its gross clinicopathologic characteristics, but its true malignant potential was probably underappreciated.45 Although in 1930, Spies first used the term adenoid cystic carcinoma,46 the term cylindroma persisted until 1942, when Dockery and Mayo suggested cylindroma-type adenocarcinoma. By then, the malignant, albeit indolent, behavior of this tumor was more widely appreciated. In fact, in their 1942 description, the propensity of ACC for “mspread along nerve sheaths” is first mentioned.47 Finally, Foote and Frazell wrote the first definitive review, detailing its classic histopathologic features.48
Clinical Presentation
It has been estimated that adenoid cystic carcinoma constitutes up to 10% of all salivary neoplasms. Although ACC is thought to be the second-most common malignant tumor of the salivary glands, it is the most common malignancy found in the minor salivary, submandibular, and sublingual glands. In two large reviews, the most common site of origin is the oral cavity, followed by the sinonasal tract.49,50 The palate is the most common location in the oral cavity (Fig. 10–5).
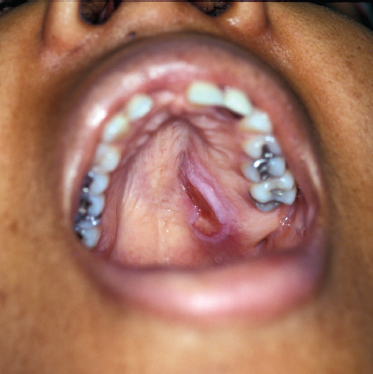
FIGURE 10-5 Adenoid cystic carcinoma presenting on the hard palate.
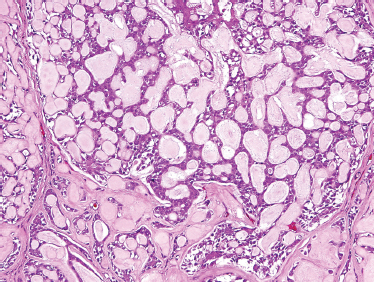
FIGURE 10-6 Adenoid cystic carcinoma: cribriform form (H&E,×20).
ACC often presents as a slowly growing and infiltrative mass. Due to the propensity for perineural invasion, patients with ACC should be specifically questioned about the function of regional and motor nerves. Formication, paresthesias, and numbness should always be recorded. Muscle fasciculation or atrophy and facial paresis are clear signs of facial nerve involvement. Regional lymphatic metastasis in ACC is not common. For major and minor salivary glands, less than 20% of patients with ACC developed metastasis.49,50 But in a review of submandibular ACC, 24% of patients were found to have regional lymphatic spread.51
Imaging
Abnormal attenuation within the pterygopalatine fossa on soft tissue CT windows52 or abnormal signal intensity/enhancement on postcontrast T1-weighted fat-suppressed MRI is strong evidence of perineural tumor spread.53 The foramen ovale and rotundum, the cavernous sinus, and Meckel’s cave should also be carefully examined with high-resolution MRI to exclude perineural dissemination.
Pathogenesis
ACC is divided based on tumor architecture into cribriform, tubular, and solid patterns. The cribriform type is most similar and best known because of its classic “Swiss cheese” morphology (Fig. 10–6). In this form, circular tumor nests are seen within pseudoglandular spaces filled with hyaline-like connective tissue. The solid pattern (Fig. 10–7) represents a high-grade tumor and is characterized by sheets of basaloid cells with minor tubular or cribriform components. The tubular pattern is considered generally a lower grade ACC (Fig. 10–8) and is composed of ductal structures formed of outer myoepithelial and inner epithelial cells. The cribriform pattern has the best prognosis, with the tubular having a prognosis intermediate to the cribriform (glandular architecture) and solid (epithelial architecture) patterns.50 Others have found prognosis more closely correlated with staging than histology. Tubular and solid patterns have been reported to have more early local recurrence than cribriform with survival rate determined more by stage.
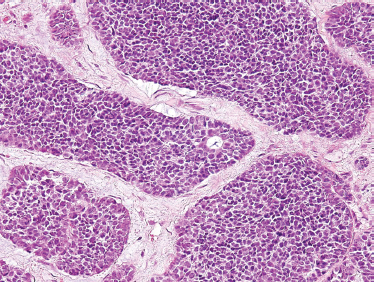
FIGURE 10-7 Adenoid cystic carcinoma: solid form (H&E,×20).
For the diagnosis of ACC, most tumors demonstrate mixed architecture, and the predominant subtype determines the diagnosis. ACC can be easily confused with basosquamous carcinoma of the upper aerodigestive tract because of the pseudocribriform pattern. This should always be considered when the diagnosis of adenoid cystic carcinoma is found within lymph node(s), especially if the primary is unknown.
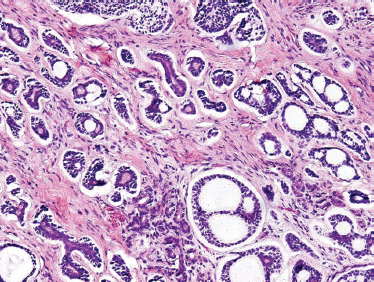
FIGURE 10-8 Adenoid cystic carcinoma (predominantly tubular form with focal cribriform areas) (H&E,×20).
The most remarkable feature of ACC is its predilection for perineural invasion. Because most ACC of the minor salivary glands arise from sites in the oral, oropharyngeal, and paranasal mucosa, the maxillary (V2) and the mandibular (V3) branches of the trigeminal nerve are most commonly involved by tumor. The facial nerve is typically involved by tumors arising in the parotid gland. For submandibular or sublingual tumors, the lingual, hypoglossal, and marginal mandibular nerves are at risk.
Perineural involvement is found often in patients with advanced or high-grade tumors and with recurrence, yet its pathogenesis remains poorly understood. Perineural invasion (PNI) is the process by which cancer cells penetrate the perineural space and permeate along the peripheral nerves. Tumor can spread along the nerve following a path of least resistance through connective tissue.
Although described by Cruveilhier in 1835,54 Ernst published the first rigorous review in 1905.55 Adenoid cystic and other salivary malignancies are not the only “neurotropic” cancers. PNI is also commonly encountered in prostate, biliary tree, and pancreatic carcinomas, as well as head and neck cutaneous and mucosal cancers. Although Ernst proposed that PNI resulted from tumoral transit through lymphatic vessels within the perineural space, Rodin et al demonstrated in prostate cancer that the perineurium was devoid of lymphatics.56 These authors proposed that tumor cells infiltrate nerves as a path of “least resistance” into the perineural space. More recently, tumor deposition of laminin-557 and nerve cell adhesion molecule expression58 have been implicated in the pathogenesis of PNI.
In the MD Anderson Cancer Center review,50 half of the patients (79 of 160) presented with perineural invasion. Major (or named) nerves were involved in 36 patients, and minor or unnamed nerves displayed evidence of perineural invasion in 43 patients. In the largest major review using current treatment approaches, major nerve involvement was clearly associated with both increased locoregional failure and diminished survival.50 Now in most centers, assessment for perineural spread is routinely performed for patients with ACC. Even so, its identification in ACC depends on the fastidiousness of histopathologic examination of tumor specimens.
Genetics
The molecular alterations that underlie the development and progression of ACC remain unknown. A recent study using oligonucleotide microarray analysis59 identified several genes that were associated with ACC. These genes included casein kinase 1, transcription factors SOX4 and AP-2 gamma, as well as members of the Wnt/β-catenin signaling pathway: epsilon and frizzled-7. Future study with genomic and proteomic assessment of expression patterns in patient tumor samples and serum will be needed to elucidate the mechanism of ACC pathogenesis.
Treatment
Adenoid cystic carcinoma is characterized by a protracted and variable clinical course. Even after successful local and regional treatment, there remains a great tendency for delayed local and distant spread.
The mainstay of treatment is surgery and postoperative radiation therapy.22 Appropriate resection of the affected salivary gland (submandibular and/or sublingual resection, parotidectomy, or wide resection of minor salivary tumor) should be performed whenever possible. Elective neck dissection is not used routinely. The surgical management of perineural invasion is discussed earlier.
Given the high incidence of PNI, patients will often require postoperative radiation therapy (see Chapter 13). Thus preoperative dental consultation is required. For tumors of the base of the tongue and other minor salivary gland tumors, postoperative radiation therapy is important in achieving final local control after gross total excision. A postoperative dose of 60 Gy in 30 fractions to the operative bed is recommended. When a major, named nerve is invaded, the path of the nerve is treated electively to its ganglion.16 The largest panoramic view of the natural history of adenoid cystic carcinoma was presented in Conley et al’s 1991 textbook.60 This review of 406 patients demonstrated that at 10 years, roughly one third of patients were free of disease, one third were dead of disease, and one third were alive with disease. Thus true incidence of loco-regional control and/or distant metastasis depends on the duration of follow-up for these patients. Conley et al60 postulated that with 30-year follow-up as many as 80% of patients would succumb to their disease. In the MD Anderson series, disease-specific survival rates later confirmed this trend. At 5 and 7 years, disease-specific survival was 89.0 and 74.8%, which dropped to 67.4% and 39.6% at 10 and 15 years, respectively.50,61
Compared with carcinomas of the upper aerodigestive tract, the survival of patients with ACC does not stabilize at 5 years, and the survival rate continues to decline even after 15 years. Depending on the number and location of the sites of distant failure, prolonged survival with the disease is possible. Accordingly, patients should be counseled that even after local and regional control is achieved, adenoid cystic carcinoma remains a chronic disease, and that yearly lifetime follow-up is required for adequate surveillance.
With appropriate local and regional treatment, the most common mode of failure is now distant metastasis. The most common sites are lung (67%), liver (12%), and bone. Although predictive indicators for distant metastasis remain elusive, clinical stage and locoregional failure are most important.50,61 Although anecdotal, numerous reports suggest an increased rate of distant failure for patients with ACC of the submandibular gland.62
There is no effective treatment for the development of distant metastasis from ACC. Half of patients have distant metastasis 3 years after diagnosis of their tumors.62 The most studied regimen for ACC is cyclophosphamide, doxorubicin, and cisplatin (CAP).32,33 Unfortunately, despite an occasional complete response, there has been no demonstrated survival benefit. Surgical resection, especially for pulmonary metastases, may be considered for isolated lesions, although no survival benefit has been shown.63,64 Almost half of patients have more than one site of failure, with distant metastasis associated with locoregional failure in two thirds of patients.64 Patients may have subclinical metastasis at the time of diagnosis of the primary tumor. Thus such palliative therapies should be reserved for symptomatic patients, with adequate performance status and with an understanding of the inexorable course of this disease.
Acinic Cell Carcinoma
Clinical Presentation
Acinic cell carcinoma accounts for/1to6%ofall salivary tumors. The parotid gland is the most often involved site, and occasionally it can present bilaterally. The most common presentation is a solid or cystic mass in the parotid gland with a slow-growing behavior. The mass can be associated with regional pain and facial dysfunction or paralysis. In adults it presents most commonly during the fourth or fifth decade of life and shows a female predominance. Acinic cell carcinoma is the second most common malignant salivary gland tumor in children.65
Imaging
CT and MRI findings are nonspecific for the disease and usually of a benign appearance. On CT, acinic cell carcinoma may show contrast enhancement with single or multiple lobules with or without calcifications. MRI is nonspecific, although if surrounding fat is displaced or obliterated, invasion is suggested.66
Histology
The classic histological appearance of acinic cell carcinoma (Fig. 10–9) displays large, polygonal serous cells with basophilic cytoplasm with coarse granules and small, centralized nuclei. These granules can be demonstrated with periodic acid-Schiff (PAS) diastase staining. Several patterns have been observed, including tubular, ductoglandular, medullary, acinar, follicular, microcystic/macrocystic, papillary cystic, and solid, with the solid and microcystic being the most common patterns.67,68 No significant prognostic predictive value is associated with subtypes,68 although some have proposed that a solid architecture is associated with a poorer prognosis.69 The supporting stroma, although small, can contain a lymphocytic spread with formation of germinal centers in this tumor type. Acinic cell carcinoma may not uncommonly develop in intraparotid lymph nodes and should be differentiated from metastatic disease.
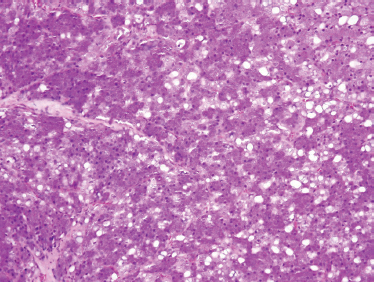
FIGURE 10-9 Acinic cell carcinoma (H&E,×20).
Genetics
Genetic alterations at certain chromosomal loci in 25 primary parotid acinic cell carcinomas have been identified. In one study, 84% of the tumors had alteration in at least one of the chromosomal loci: 4p, 5q, 6p, and 17p predominated over 1p and 1q, 4q, 5p, and 6q. Loss of heterozygosity (LOH) at 4p15–16, 6p25-qter, and 17p11 suggests the possibility of tumor suppressor genes involved in tumorigenesis of acinic cell carcinoma. In addition, LOH was significantly associated with tumor grade.70
Treatment
In most cases, acinic cell carcinoma behaves as a relatively low-grade malignancy. As such, adequate surgical resection will yield acceptable rates of local control and overall survival.65,71 Despite reports of associated adjacent microscopic, lymphocytic infiltration, these tumors metastasize in only 10 to 20% of cases.71 Thus adequate surgical margins are paramount, as local recurrence is common with incomplete or close excisions. Postoperative radiation seems to be effective in treating microscopic disease remaining after surgery.72 Local recurrence occurs in one third of patients.71 Distant failure, though rare, has been reported many years after initial treatment, most commonly to the lungs and bone.65 Negative prognostic factors, pain, and facial nerve dysfunction occur in 5 to 10% of patients. Other negative prognosticators include deep lobe tumors, multiple nodules, and distant metastasis.71
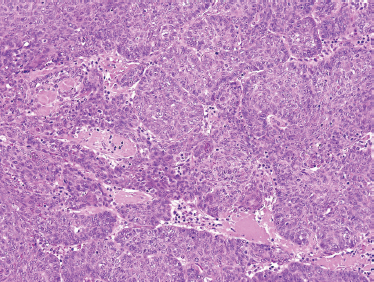
FIGURE 10-10 Basal cell salivary adenocarcinoma: nests of uniform basal cells with peripheral palisading (H&E,×20).
Basal Cell Salivary Adenocarcinoma
Clinical Presentation
Even among salivary gland neoplasms, basal cell adenocarcinoma is quite rare. Among the reported cases, 90% are found in the parotid salivary gland, although they may present in the submandibular and minor salivary glands.73 These tumors typically present in the sixth decade74 as a long-standing, asymptomatic mass, with sudden growth and associated pain.
Histology
Basal cell salivary adenocarcinoma should be differentiated from solid-pattern adenoid cystic carcinoma and metastatic basal cell carcinoma of skin. It is comprised of small ovoid cells with dark hyperchromatic nuclei with scant cytoplasm (Fig. 10–10) and occasionally polygonal cells with eosinophilic cytoplasm. Peripheral palisading can be seen as well (Fig. 10–11). Solid, membranous, tubular, and trabecular forms have been reported.
Basal cell salivary adenocarcinoma may arise de novo or as a carcinoma ex-basal cell adenoma (Fig. 10–12). An infiltrative pattern of growth distinguishes this neoplasm from basal cell adenoma. However, invasion must be carefully defined and distinguished from both multinodular and multifocal disease, both of which are features of basal cell adenoma.75 Both perineural and lymphovascular invasion are not uncommon, especially in tumors arising from the minor salivary glands.
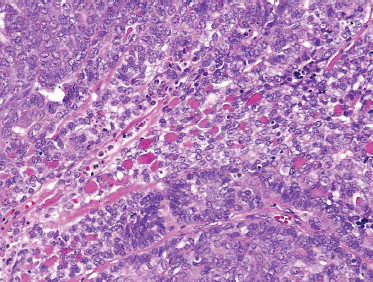
FIGURE 10-11 Basal cell salivary adenocarcinoma (H&E, ×40).
The differential diagnosis includes an impressive array of similar histological species. Basal cell adenocarcinoma must be distinguished from basal cell adenoma on the basis of extraglandular extension and the infiltrative nature of the adenocarcinoma. Basal cell adenocarcinoma can closely resemble solid pattern adenoid cystic carcinoma in the homogeneity of dark tumor cells, though without the variegation typically seen in adenoid cystic carcinoma. In addition, a higher mitotic rate and more necrosis are more often seen in ACC. Basal cell adenocarcinoma can be distinguished from terminal duct/polymorphous low-grade adenocarcinoma by the basaloid, uniform architecture and cellular composition, in contrast to the greater cellular variability typically seen in terminal duct adenocarcinoma. Undifferentiated small cell or neuroendocrine carcinomas and basaloid-squamous cell carcinoma can also be included in the differential diagnosis.
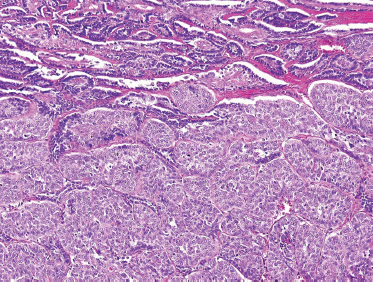
FIGURE 10-12 Basaloid salivary adenocarcinoma (lower two thirds) ex-monomorphic adenoma (upper one third) (H&E,×20).
Basal cell adenocarcinoma tends to act as a lower-grade malignancy. In the parotid and submandibular glands, with proper surgical resection and appropriate margins, local recurrence, as well as regional and distant spread, is rare. However, for tumors arising in minor salivary glands, radiotherapy may be required when there is evidence of an infiltrative growth pattern, or with evidence of perineural and vascular invasion. As with any malignancy with a low mitotic rate, patients with basal cell adenocarcinoma should be followed on a long-term basis, as delayed occult recurrence remains a possibility.74,75
Myoepithelial Carcinomas
The myoepithelial cell is believed to arise from the ectodermal precursor of the intercalated duct and was first described by Zimmerman in 1898.76 As such, it is an integral component of several salivary tumors with both mesenchymal and epithelial features.77 Yet myoepithelial carcinoma is exceedingly rare.
Clinical Features
Myoepithelial carcinomas are unencapsulated, infiltrative tumors predominantly found in the parotid gland. Myoepithelial carcinoma may arise de novo or within a long-standing pleomorphic adenoma. Di Palma and Guzzo78 considered myoepithelial carcinoma to be low grade when it arises in a pleomorphic adenoma, and high grade when it arises de novo. However, a larger and more recent review did not find this to be the case.79 Comprehensive surgical resection with wide margin is necessary for adequate local control. Regional metastasis is uncommon, but distant failure is well documented in the few large reviews available.78,79
Histology
The tumor is composed of myoepithelial cells lacking any ductal or acinar differentiation80,81 and should be distinguished from the benign monomorphic “myoepithelioma” and myoepithelial-predominant pleomorphic adenoma. Myoepithelial carcinoma may present as pure spindle cell, pure plasmacytoid, and a mixture of the two. Both spindled and plasmacytoid forms (Fig. 10–13) manifest malignant cellular features, including pleomorphism, high rates of mitosis, and necrosis. Immunohistochemical staining for smooth muscle actin and S-100 (Fig. 10–14) is typically positive. Also, strong epithelial membrane antigen (EMA) and/or cytokeratin staining confirm the epithelial nature of this lesion. Based on careful immunohistochemical assessment (Table 10–3), the diagnosis of myoepithelial carcinoma can be established by their characteristic positivity for cytokeratin, S-100, and smooth muscle actin.
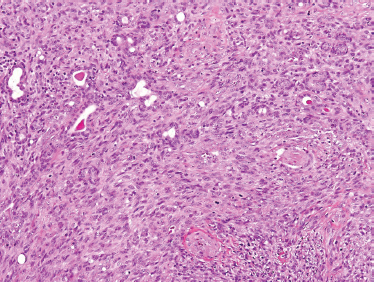
FIGURE 10-13 Myoepithelial carcinoma: spindle cell form (H&E,×20).
The tumor should also be distinguished from leiomyosarcoma, nerve sheath tumor, synovial sarcoma (which has distinctive biphasic features), and spindle cell melanoma. Clear cell and epithelial (Fig. 10–15) variants are described but should be distinguished from epithelial-myoepithelial carcinoma (see below).
Salivary Clear Cell Carcinomas
Primary clear cell tumors represent a richly diverse category of salivary gland malignancy.82 Primary salivary carcinomas with clear cell features include epithelial-myoepithelial carcinoma (EMEC) and clear cell carcinoma (CCC).83 As discussed above, myoepithelial carcinoma has features of clear cell carcinoma as well. However, there are also clear cell variants of other salivary tumors, including acinic cell carcinoma, oncocytoma, and mucoepidermoid carcinoma, which must always be considered. Finally, within the head and neck, metastatic tumors, such as renal cell carcinoma and balloon cell melanoma, should also be considered.
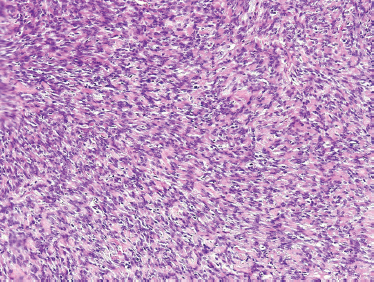
FIGURE 10-14 Myoepithelial carcinoma: spindle cells with palisading features) (H&E,×20).
Epithelial-Myoepithelial Carcinoma
Epithelial-myoepithelial carcinoma was first proposed by Donath et al84 in 1972, although similar tumors had been described previously as clear cell carcinoma, clear cell adenoma, glycogen-rich adenoma, and glycogen-rich adenocarcinoma. Corio et al85 translated the term and introduced this neoplasm into the English-language medical literature.
Epithelial-myoepithelial carcinomas are most commonly found within the parotid glands (75%),83,85–88 and the rest occur about evenly in the submandibular and sublingual glands and the minor salivary glands. It has predilection to occur in women, by a ratio of nearly 2:1. Because of their infiltrative pattern of growth, EMEC displays a particularly decided locoregional aggressiveness, but, when appropriately treated with complete surgical resection, it has a relatively low mortality.
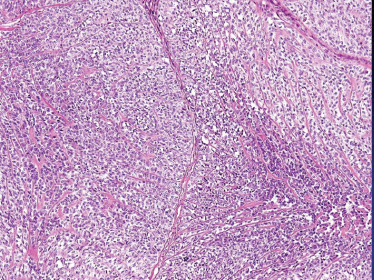
FIGURE 10-15 Myoepithelial carcinoma: epitheloid cellular features (H&E,×20).
The distinguishing histological feature of EMEC is classically described as sheets of well-delineated tubules lined by two layers; the internal layer with prominently eosinophilic ductal cells, and the outer layer with pale clear cells of myoepithelial origin with abundant cells overlying a basement membrane (Fig. 10–16). Both cell types vary in their distribution and phenotypic expression, not only from patient to patient, but also within the same tumor. However, typically the clear cells of the outer tubular layer are polyhedral (Fig. 10–17) and contain abundant diastase-digestible PAS-positive cytoplasmic granules, which stain negative for mucicarmine. Often, there is minimal nuclear pleomorphism and low rates of mitotic figures. Though rare, EMEC has such a distinctive histological appearance that it should be considered in the differential cytological diagnosis of any biphasic tumor.82
Clear Cell Carcinoma
Clear cell carcinoma is characterized by a predominant distribution of tumor cells with clear, PAS-positive cytoplasm. The tumor cells are cuboidal to polygonal with high nuclear-cytoplasmic ratios. CCC appears more homogenous when compared with EMEC with more cellular uniformity. Focal areas of eosinophilic cytoplasm and/or squamous differentiation can also be found. Glandular ductal formations are not typically seen.82
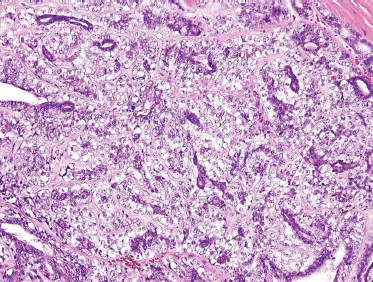
FIGURE 10-16 Epimyoepithelial carcinoma: low power (H&E,×20). (Note: dark duct epithelial and clear periductal myoepithelial cells.)
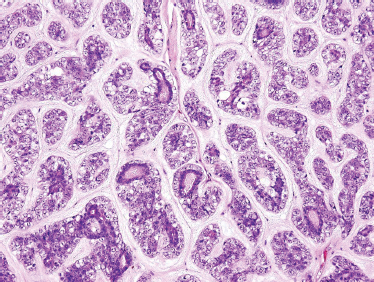
FIGURE 10-17 Epimyoepithelial carcinoma: high power (H&E,×40).
Salivary Duct Carcinoma
Clinical Presentation
Salivary duct carcinoma (SCD) is a rare, high-grade epithelial malignancy with a remarkable resemblance to mammary ductal carcinoma. The most commonly involved sites are the parotid gland in more than 85% of the cases, followed by the submandibular gland. The clinical presentation of patients with SDC is either a painless or pain-associated swelling of the parotid gland. Concurrent symptoms may include facial nerve dysfunction or paralysis in as many as a third of patients. There is a clear male predominance, and the age range is usually greater than 50 years old.
Histology
“Speichelgangcarcinoma” was first described in 1968 by Kleinsasser et al89 and later translated into salivary duct carcinoma.90,91 Cells are cuboidal or polygonal with ductal formations and comedonecrosis. They contain amphophilic and eosinophilic cytoplasm with a granular or powder-like interior. Nuclear pleomorphism as well as cellular variability can be seen with the presence of mitotic figures. Salivary duct carcinoma typically displays intraductal and infiltrative ductal features (Figs. 10–18, 10–19). They are often associated with desmoplastic reaction in the surrounding soft tissues. They may present as de novo or most commonly in the setting of carcinoma ex-pleomorphic adenoma.
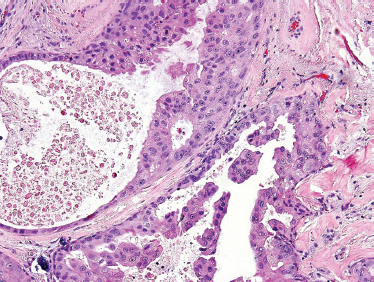
FIGURE 10-18 Salivary duct carcinoma: papillary formation (H&E, × 40).
Although solid areas may be seen, the most common histological patterns include the comedonecrotic, cystic (Fig. 10–20), papillary, and cribriform subtypes. Goblet cell metaplasia, basal cell hyperplasic, and background necrosis can be seen adjacent to the area of the neoplasia. Perivascular and perineural invasion commonly occurs. Extensive perineural invasion and lymphovascular invasion are common.
Treatment
SCD is a high-grade malignancy with high rates of early metastasis and death. Parotidectomy with appropriate neck dissection is the mainstay of surgical treatment. Because ~60% of patients will have regional lymphatic spread, an appropriate cervical lymphadenectomy should always be considered. Postoperative radiation is advocated for this aggressive malignancy.
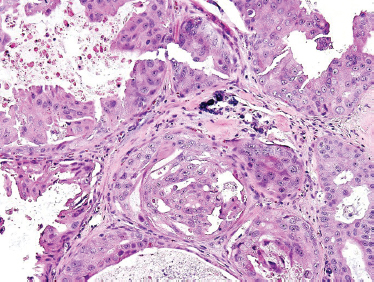
FIGURE 10-19 Salivary duct carcinoma (H&E,×40).
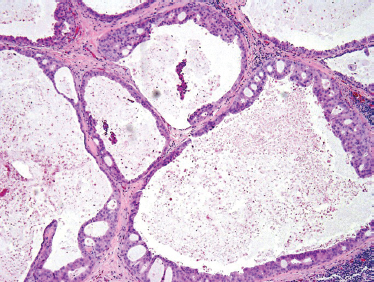
FIGURE 10-20 Salivary duct carcinoma (cystic) (H&E, × 20).
Despite appropriate surgery and postoperative radiation therapy, many patients succumb to distant disease. In fact, distant metastasis is common, even at primary presentation, to the lungs, bone, liver, and brain. In the three largest clinical reviews of the disease, the rate of distant metastasis varied from 46 to 62% and was often the cause of death. Therefore, a thorough preoperative workup should be undertaken. Although no proven systemic therapy is available for patients with salivary duct carcinoma, transtuzumab may provide hope for systemic therapy for patients with SDC (see previous discussion).31
Terminal Duct (or Polymorphous Low-Grade) Adenocarcinoma
Clinical Presentation
Terminal duct adenocarcinoma (TDC) or polymorphous low-grade adenocarcinoma is the second most common malignant salivary tumor in the oral cavity. The most common sites are the buccal mucosa and at the junction of the soft and hard palate, usually in elderly individuals. Other sites include lip and retromolar trigone. Patients typically present with a painless, elevated, and firm mass, usually with an intact mucosa around it. The occasional patient presents with pain or a poor fitting denture. Because of the overall good prognosis, pain is not a negative prognostic factor, as can be seen with other salivary gland malignancies. Some clinicopathological reports have shown a slight predominance in females.92
Although TDC is well known for its predilection to arise from minor salivary glands, these tumors are also diagnosed in the parotid gland. One review of 22 TDCs of parotid gland confirmed the low-grade nature of these tumors, regardless of the site of origin.93 TDC shares with adenoid cystic carcinoma a similar histogenesis (intercalated duct origin) and cellular composition. Not surprisingly, the same pattern of local and perineural invasion is seen in both tumors.94 In the past, it has been called lobular carcinoma or trabecular carcinoma of the salivary glands.
Histology
TDC is characterized by cuboidal or columnar cells of small to medium size, with an ovoid to spindle nucleus with fine chromatin and small nucleoli. The scant eosinophilic cytoplasm is mostly clear, although at times it may present with a granular or mucoid appearance. Mitotic figures, nuclear atypia, and necrosis are absent features. The benign morphology of the cells may confuse them with a monomorphic adenoma.
There are four typical growth patterns: solid (Fig. 10–21), cribriform, cystic, and tubular, and combinations thereof (Fig. 10–22). The tumor may be circumscribed but unencapsulated. The stroma may be mucoid, hyaline, or mucohyaline and loosely vascular. Invasion into the surrounding tissues typically presents either by single or multiple ducts and by solid epithelial nodules. Extension to the adjacent bone (hard palate) may also be observed. Some of the cells show a spindle cell appearance that could be suggestive of myoepithelial differentiation.94
Treatment
Complete surgical excision is the preferred mode of treatment.92,93 Neck dissection is rarely indicated, as involvement of regional nodes is well below 10%. With appropriate surgical margins, local recurrence is low, but it has been reported to be as high as 17%.95 Mortality occurs in 5 to 10% of cases. Due to the high incidence of PNI, these patients may be candidates for adjuvant postoperative radiotherapy; however, no survival advantage has been proven.
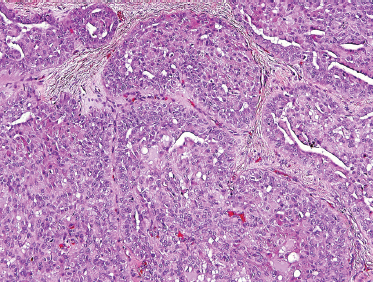
FIGURE 10-21 Terminal duct carcinoma solid component (H&E,×40).
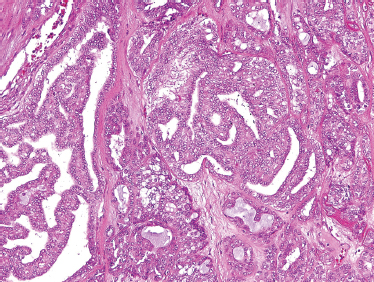
FIGURE 10-22 Terminal duct carcinoma (cystic and tubular components) (H&E,×40).
Unclassified Adenocarcinoma
Adenocarcinoma not otherwise specified (NOS) of salivary tissue is a category used with diminishing frequency and now is most often used as a diagnosis of exclusion. Clinicopathological entities such as salivary duct carcinoma, terminal duct carcinoma, and epimyoepithelial carcinoma, formerly in the NOS category, are examples of this process. There remain, however, adenocarcinomas of salivary tissues that cannot be accommodated in conventional classifications.96 They are the least common of salivary carcinomas (1–2% of tumors) and manifest a cytoarchitecture ranging from a well-differentiated, low-grade appearance to high-grade, invasive lesions (Fig. 10–23).
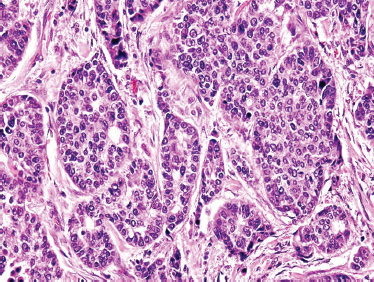
FIGURE 10-23 Adenocarcinoma not otherwise classified (H&E, × 40).
 Epithelial-Mesenchymal-derived Salivary Gland Malignancies
Epithelial-Mesenchymal-derived Salivary Gland Malignancies
These tumors conceptually derive from both epithelial and mesenchymal salivary gland tissues and include carcinoma ex-pleomorphic adenoma, carcinosarcoma, and metastasizing pleomorphic adenoma.
Carcinoma Ex-Pleomorphic Adenoma
Carcinoma ex-pleomorphic adenoma (CXPA) is the most common “malignant mixed” tumor. It has been estimated that CXPA may account for as much as 10% of all salivary gland malignancy. It arises from long-standing benign pleomorphic adenoma (mixed tumor) or in the setting of recurrent disease, mostly in the parotid gland. A typical presentation is the rapid growth of tumor in a long-standing salivary mass. The risk of malignant transformation increases with time and age of the patient. Among younger patients with pleomorphic adenoma, their lifetime risk may be higher for malignant transformation. Thackray and Lucas97 estimated that malignant transformation eventually occurred in up to 25% of untreated mixed tumors. In an epidemiological study of 498 patients, Spitz et al4 estimated at least a 3 to 10% rate of conversion from benign to malignant disease tumors. From a classic 1974 study, it has been estimated that 1 to 2% of adenomas of less than 5 years’ duration, and 9.4% of adenomas present for more than 15 years, can transform into CXPA.98
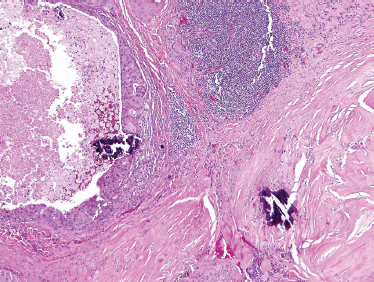
FIGURE 10-24 Carcinoma ex-pleomorphic adenoma: hyalinized and fibrotic remnants of pleomorphic adenoma: lower right (H&E, × 20).
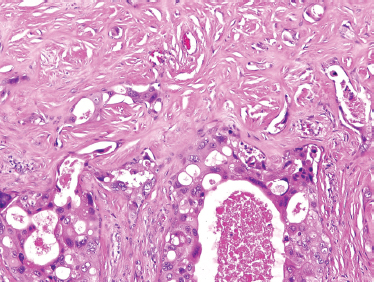
FIGURE 10-25 Salivary duct carcinoma ex-pleomorphic adenoma (H&E, × 40).
Histology
These tumors are comprised only of epithelial-derived carcinoma arising within the mixed tumor tissue types. CXPA often displays carcinoma within residual foci of benign mixed tumor (Fig. 10–24). If metastasis is present, only the carcinomatous element is present. In order of descending frequency, the carcinomatous histological subtypes seen within CXPA are salivary duct (Fig. 10–25), poorly or undifferentiated (Fig. 10–26) carcinoma, terminal duct carcinoma, myoepithelial carcinoma, and adenocarcinoma NOS. However, a thorough sampling of the entire tumor specimen is necessary to identify the preexisting benign component. Occasionally, the pleomorphic adenoma can be entirely replaced by a hyalinized round nodule, confounding diagnosis. An infiltrative growth pattern, hyalinization, hemorrhage, and calcification are often seen. Because the residual mixed tumor may be small, misdiagnosis is possible.
Immunohistochemical evidence suggests the antigens laminin and collagen IV, the main constituents of the basement membrane, may be involved in the malignant transformation of mixed tumors. The basement membrane is involved in cell differentiation and proliferation. Disruption of the basement membrane has been associated with the invasive properties of neoplasms. The discovery of significantly greater collagen IV deposits around tumor cell aggregates from metastasizing as opposed to nonmetastasizing carcinoma ex-pleomorphic adenoma suggests a role of collagen IV in the biological progression of the disease.99 Carcinoembryonic antigen expression and high proliferative activity are associated with malignant transformation of the mixed tumor.100
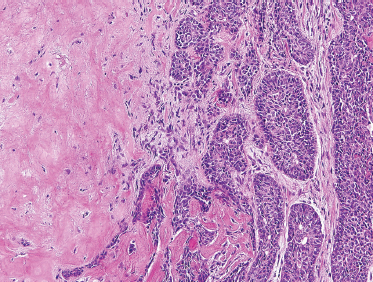
FIGURE 10-26 Poorly differentiated carcinoma (right) ex-pleomorphic adenoma (left) (H&E, × 20).
Treatment
These aggressive tumors are generally treated with surgery and radiation therapy. The 42% rate of local recurrence in the rare submandibular and minor salivary gland CXPA is twice the rate of local recurrence in the parotid. Cervical metastasis occurs in 10 to 20% of CXPA cases. Distant metastasis occurs in 30% of patients, with the lung and bone most commonly involved. Long-term survival rates of frankly invasive CXPA are 30 to 40%. This drops to 3% in patients with nodal disease.101 A subset of CXPA patients with microscopic invasion of less than 8 mm has a potentially favorable prognosis.102
Carcinosarcoma
For the diagnosis of carcinosarcoma, both the primary tumor and any metastasis must display evidence of both malignant epithelial and malignant mesenchymal components. As such, this tumor is exceedingly rare and poorly understood. Typically, carcinosarcoma presents suddenly within a long-standing mixed tumor of the major salivary glands, particularly the parotid. Its natural history is fulminant, aggressive, and often rapidly fatal.
Histology
A biphasic microscopic morphology must be identified to establish the diagnosis of carcinosarcoma. The spectrum of sarcomas includes chondro-, fibro-, leio-, myxo-, and osteosarcoma, as well as malignant fibrous histiocytoma, most often in association with a salivary ductal carcinoma (Fig. 10–27). Immunohistochemical analysis has suggested that both elements are derived from a common precursor cell, possibly of myoepithelial origin.103
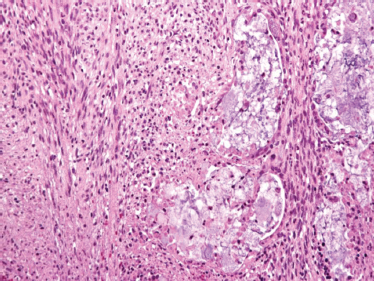
FIGURE 10-27 Carcinosarcoma: carcinoma component (right): sarcoma component (left) (H&E, × 40).
Metastasizing Pleomorphic Adenoma
Though benign on histology, metastasizing pleomorphic adenoma (mPA) is a salivary gland tumor that behaves with unequivocally malignant features, primarily in its capacity for distant metastasis. By histology and cytology, mPA cannot be distinguished from typical pleomorphic adenoma. The diagnosis can only be made after the histologically bland pleomorphic adenoma has spread to a distant site.104
Although initially referred to as a “benign” metasta sizing mixed tumor in the 1940s,105,106 subsequent reports have confirmed its functionally morbid, sometimes protracted, and even lethal107 natural history. However, this tumor must be distinguished from carcinoma ex-pleomorphic adenoma and carcinosarcoma by the lack of malignant epithelial and/or mesenchymal components, respectively.108
Many reports clearly suggest an association with previous surgical exploration. It may be that repeated surgical treatment provides an opportunity for hematogenous dissemination of an otherwise benign, and if properly treated, indolent neoplasm.109 However, this is clearly a spectrum of behavior, and these lesions must be distinguished from locally recurrent pleomorphic adenoma.110,111 With proper initial surgical treatment, rates of local recurrence should be quite low.112
The underlying mechanism for the metastatic behavior of this histologically benign tumor remains unclear. El-Naggar et al113 have suggested that metastasizing pleomorphic adenomas may represent unrecognized true malignancy. Further evidence of the clinical malignancy of some of these cases can also be drawn from previously documented cases in which ~50% of patients had died of their disease with documented metastases in bones, lungs, lymph nodes, or deep organs. The lack of recognized histological parameters of malignancy in these cases raises the possibility that a small subset of pleomorphic adenomas with submicroscopic alterations may pursue a malignant course. To date, no correlation has been found in any study between the presence or deletion of specific chromosomal alterations and subsequent tumor recurrence or malignant transformation.
Staging
The American Joint Committee on Cancer (AJCC) and the World Health Organization (WHO) provide a staging system for major salivary gland malignancy (Tables 10–4, 10–5).114 A staging system for minor salivary gland tumors does not exist.
 Mesenchymal-derived Salivary Malignancies
Mesenchymal-derived Salivary Malignancies
Primary salivary gland sarcomas represent less than 1% of salivary malignancies. They present in an older population and less commonly than benign mesenchymal-derived salivary tumors. Eighty percent of mesenchymal-derived salivary malignancies occur in the parotid as a nodule or swelling. Primary origin must be supported with sarcoma in no other site, exclusion of sarcoma invading from adjacent soft tissue, and exclusion of carcinosarcoma. Anaplastic carcinoma and neurotropic melanomas must be distinguished.115 Immunohistochemistry has an important role in histopathologic classification.
The most common are hemangiopericytoma (16% of cases), not surprising given the propensity for parotid involvement by benign vascular tumors. Malignant schwannomas (mostly spindle cell) make up 15% of cases. The abundant peripheral nerve supply in salivary glands accounts for the relatively high frequency of both benign and malignant neural tumors. Fibrosarcoma and malignant fibrous histiocytoma represent 14% and 11% of reported sarcomas, respectively.115 Nearly all forms of sarcoma present in salivary glands.
Table 10-4 2002 AJCC TNM Definitions for Staging of Major Salivary Malignancies
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Tumor 2 cm or less |
| T2 | Tumor 2 cm |
| T3 | Tumor more than 4 cm or extraparenchymal extension |
| T4a | Tumor invades skin, mandible, ear canal, or facial nerve |
| T4b | Tumor invades skull base, pterygoid plated, or encases carotid artery |
| Nx | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in a single ipsilateral lymph node 3 cm or less |
| N2a | Metastasis in single ipsilateral lymph node 3 to 6 cm |
| N2b | Metastasis in multiple ipsilateral lymph nodesB6cm |
| N2c | Metastasis in bilateral or contralateral lymph nodesB6cm |
| N3 | Metastasis in lymph node>6cm |
| Mx | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
AJCC, American Joint Committee on Cancer
Table 10-5 2002 AJCC Stage Groupings for Major Salivary Malignancies
| Stage 1 | T1N0M0 |
| Stage 2 | T2N0M0 |
| Stage 3 | T3N0M0, T1N1M0, T2N1M0, T3N1M0 |
| Stage 4a | T4aN0M0, T4aN1M0, T1N2M0, T2N2M0, T3N2M0, T4aN2M0 |
| Stage 4b | T4b any N M0, any TN3M0 |
| Stage 4c | Any T, any N M1 |
AJCC, American Joint Committee on Cancer
The treatment is aggressive surgical resection and postoperative radiation therapy. Recurrences manifest in 40 to 64% of patients, with metastases (usually hematogenous) in 38 to 64%. Metastasis is most common to the lung, followed by cervical lymph nodes. A rate under 10% for cervical metastasis suggests elective neck dissection is not necessary. Mortality is 36 to 64% within 3 years of diagnosis, with the average time from treatment to death being 2.4 years.115,116 Tumors arising within the gland have fewer recurrences and metastases than in patients with secondary salivary gland involvement. This may well be more related to the stage of the disease rather than the site of location.115 Salivary gland sarcomas behave like their soft tissue counterparts with prognosis related to tumor size, sarcoma type, and histological grade.116 Kaposi’s sarcoma presents in acquired immunodeficiency syndrome (AIDS) patients usually in intraparotid nodes.
REFERENCES
1. Hoffman, HT Karnell, LH Robinson, RA Pinkston, JA Menck, HR. National Cancer Data Base report on cancer of the head and neck: acinic cell carcinoma. Head Neck 1999; 21 (4): 297–309
2. Jessup, JM Menck, HR Winchester, DP Hundahl, SA Murphy, GP. The National Cancer Data Base report on patterns of hospital reporting. Cancer 1996; 78 (8): 1829–1837
3. Pinkston, JA Cole, P. Incidence rates of salivary gland tumors: results from a population-based study. Otolaryngol Head Neck Surg 1999; 120 (6): 834–840
4. Spitz, MR Tilley, BC Batsakis, JG Gibeau, JM Newell, GR. Risk factors for major salivary gland carcinoma: a case-comparison study. Cancer 1984; 54 (9): 1854–1859
5. Spitz, MR Batsakis, JG. Major salivary gland carcinoma: descriptive epidemiology and survival of 498 patients. Arch Otolaryngol 1984; 110 (1): 45–49
6. Spitz, MR Fueger, JJ Goepfert, H Newell, GR. Salivary gland cancer: a case-control investigation of risk factors. Arch Otolaryngol Head Neck Surg 1990; 116 (10): 1163–1166
7. Belsky, JL, Tachikawa, K Cihak, RW Yamamoto, T. Salivary gland tumors in atomic bomb survivors, Hiroshima–Nagasaki, 1957 to 1970. JAMA 1972; 219 (7): 864–868
8. Schneider, AB Favus, MJ Stachura, ME Arnold, MJ Frohman, LA. Salivary gland neoplasms as a late consequence of head and neck irradiation. Ann Intern Med 1977; 87 (2): 160–164
9. Zheng, R Wang, LE Bondy, ML Wei, Q Sturgis, EM. Gamma radiation sensitivity and risk of malignant and benign salivary gland tumors: a pilot case-control analysis. Cancer. 2004; 100 (3): 561–567
10. Eversole, LR. Histogenic classification of salivary tumors. Arch Pathol 1971; 92 (6): 433–443
11. Dardick, I. Mounting evidence against current histogenetic concepts for salivary gland tumorigenesis. Eur J Morphol 1998; 36 (Suppl): 257–261
12. Batsakis, JG. Salivary gland neoplasia: an outcome of modified morphogenesis and cytodifferentiation. Oral Surg Oral Med Oral Pathol 1980; 49 (3): 229–232
13. Batsakis, JG Regezi, JA Luna, MA El-Naggar, A. Histogenesis of salivary gland neoplasms: a postulate with prognostic implications. J Laryngol Otol 1989; 103 (10): 939–944
14. Hicks, MJ El-Naggar, AK Flaitz, CM Luna, MA Batsakis, JG. Histocytologic grading of mucoepidermoid carcinoma of major salivary glands in prognosis and survival: a clinicopathologic and flow cytometric investigation. Head Neck 1995; 17 (2): 89–95
15. Borthne, A Kjellevold, K Kaalhus, O Vermund, H. Salivary gland malignant neoplasms: treatment and prognosis. Int J Radiat Oncol Biol Phys 1986; 12 (5): 747–754
16. Garden, AS Weber, RS Ang, KK Morrison, WH Matre, J Peters, LJ. Postoperative radiation therapy for malignant tumors of minor salivary glands: outcome and patterns of failure. Cancer 1994; 73 (10): 2563–2569
17. Garden, AS El-Naggar, AK Morrison, WH Callender, DL Ang, KK Peters, LJ. Postoperative radiotherapy for malignant tumors of the parotid gland. Int J Radiat Oncol Biol Phys 1997; 37 (1): 79–85
18. Storey, MR Garden, AS Morrison, WH Eicher, SA Schechter, NR Ang, KK. Postoperative radiotherapy for malignant tumors of the submandibular gland. Int J Radiat Oncol Biol Phys 2001; 51 (4): 952–958
19. Guillamondegui, OM Byers, RM Luna, MA Chiminazzo, H Jr Jesse, RH Fletcher, GH. Aggressive surgery in treatment for parotid cancer: the role of adjunctive postoperative radiotherapy. Am J Roentgenol Radium Ther Nucl Med 1975; 123 (1): 49–54
20. Armstrong, JG Harrison, LB Spiro, RH Fass, DE Strong, EW Fuks, ZY. Malignant tumors of major salivary gland origin: a matchedpair analysis of the role of combined surgery and postoperative radiotherapy. Arch Otolaryngol Head Neck Surg. 1990; 116 (3): 290–293
21. Fu, KK Leibel, SA Levine, ML Friedlander, LM Boles, R Phillips, TL. Carcinoma of the major and minor salivary glands: analysis of treatment results and sites and causes of failures. Cancer 1977; 40 (6): 2882–2890
22. Garden, AS Weber, RS Morrison, WH Ang, KK Peters, LJ. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int J Radiat Oncol Biol Phys 1995; 32 (3): 619–626
23. Vikram, B Strong, EW Shah, JP Spiro, RH. Radiation therapy in adenoid-cystic carcinoma. Int J Radiat Oncol Biol Phys 1984; 10 (2): 221–223
24. Harrison, LB Armstrong, JG Spiro, RH Fass, DE Strong, EW. Postoperative radiation therapy for major salivary gland malignancies. J Surg Oncol 1990; 45 (1): 52–55
25. King, JJ Fletcher, GH. Malignant tumors of the major salivary glands. Radiology 1971; 100 (2): 381–384
26. Wang, CC Goodman, M. Photon irradiation of unresectable carcinomas of salivary glands. Int J Radiat Oncol Biol Phys 1991; 21 (3): 569–576
26a. Griffin, TW Pajak, TF Laramore, GE Duncan, W Richter, MP Hendrickson, FR Maor, MH. Neutron vs. photon irradiation of inoperable salivary gland tumors: results of an RTOG-MRC Cooperative Randomized Study. Int J Radiol Oncol Biol Phys 1988; 15 (5): 1085–1090
27. Douglas, JG Koh, WJ Austin-Seymour, M Laramore, GE. Treatment of salivary gland neoplasms with fast neutron radiotherapy. Arch Otolaryngol Head Neck Surg 2003; 129 (9): 944–948
28. Lindsley, KL Cho, P Stelzer, KJ, et al. Clinical trials of neutron radiotherapy in the United States. Bull Cancer Radiother 1996; 83 (Suppl): 78s–86s
29. Douglas, JG Laramore, GE Austin-Seymour, M Koh, W Stelzer, K Griffin, TW. Treatment of locally advanced adenoid cystic carcinoma of the head and neck with neutron radiotherapy. Int J Radiat Oncol Biol Phys 2000; 46 (3): 551–557
30. Licitra, L Grandi, C Prott, FJ Schornagel, JH Bruzzi, P Molinari, R. Major and minor salivary glands tumours. Crit Rev Oncol Hematol 2003; 45 (2): 215–225
31. Glisson, B Colevas, AD Haddad, R, et al. HER2 expression in salivary gland carcinomas: dependence on histological subtype. Clin Cancer Res 2004; 10 (3): 944–946
32. Licitra, L Marchini, S Spinazze, S, et al. Cisplatin in advanced salivary gland carcinoma: a phase II study of 25 patients. Cancer 1991; 68 (9): 1874–1877
33. Licitra, L Cavina, R Grandi, C, et al. Cisplatin, doxorubicin and cyclophosphamide in advanced salivary gland carcinoma: a phase II trial of 22 patients. Ann Oncol 1996; 7 (6): 640–642
34. Prenzel, N Fischer, OM Streit, S Hart, S Ullrich, A. The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr Relat Cancer 2001; 8 (1): 11–31
35. Seshadri, R Firgaira, FA Horsfall, DJ McCaul, K Setlur, V Kitchen, P. Clinical significance of HER-2/neu oncogene amplification in primary breast cancer: the South Australian Breast Cancer Study Group. J Clin Oncol 1993; 11 (10): 1936–1942
36. Slamon, DJ Leyland-Jones, B Shak, S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001; 344 (11): 783–792
37. Volkmann, R. Über endotheliale Geschwü lste zugleich ein Beitrag zu den Speicheldrüsen und Gaumentumoren. Dtsch Z Chir 1895; 41: 1
38. Stewart, FW Foote, FW Becker, WF. Mucoepidermoid tumors of the salivary glands. Ann Surg 1945; 122: 820–844
39. Healey, WV Perzin, KH Smith, L. Mucoepidermoid carcinoma of salivary gland origin: classification, clinical-pathologic correlation, and results of treatment. Cancer 1970; 26 (2): 368–388
40. Batsakis, JG Luna, MA. Histopathologic grading of salivary gland neoplasms: I. Mucoepidermoid carcinomas. Ann Otol Rhinol Laryngol 1990; 99 (10, Pt 1): 835–838
41. Chan, JK Saw, D. Sclerosing mucoepidermoid tumour of the parotid gland: report of a case. Histopathology 1987; 11 (2): 203–207
42. Fadare, O Hileeto, D Gruddin, YL Mariappan, MR. Sclerosing mucoepidermoid carcinoma of the parotid gland. Arch Pathol Lab Med 2004; 128 (9): 1046–1049
43. Robin, C. Mémoires sur trois productions morbides décrites. In: Comptes rendus des séances et mémoires de la Société de Biologie. 1853: 185–196
44. Lorain, P Robin, C. Memoire sur deux nouvelles observations de tumeurs heteradeniques et sur la nature du tissu qui les compose. In: Comptes rendus des séances et mémoires de la Société de Biologie. 1854; 6: 209–221
45. Billroth, T. Die Cylindergeschwulst (cylindroma) in Untersuchungen uber die Entwicklung der Blutgerfarse, nebst Beobachtungen aus der königlichen chirurgischen Universitats-Kilinik zu Berlin. Berlin: G. Reimer; 1856: 55–69
46. Spies, W. Adenoid cystic carcinomas. Arch Surg 1930; 21: 365–404
47. Dockerty, MB Mayo, CW. Tumors of the submaxillary gland with special reference to mixed tumors. Surg Gynecol Obstet 1942; 74: 1033–1045
48. Foote, FW Jr Frazell, EL. Tumors of the major salivary glands. Cancer 1953; 6 (6): 1065–1133
49. Spiro, RH Huvos, AG Strong, EW. Adenoid cystic carcinoma of salivary origin: a clinicopathologic study of 242 cases. Am J Surg 1974; 128 (4): 512–520
50. Fordice, J Kershaw, C El-Naggar, A Goepfert, H. Adenoid cystic carcinoma of the head and neck: predictors of morbidity and mortality. Arch Otolaryngol Head Neck Surg. 1999; 125 (2): 149–152
51. Weber, RS Byers, RM Petit, B Wolf, P Ang, K Luna, M. Submandibular gland tumors: adverse histologic factors and therapeutic implications. Arch Otolaryngol Head Neck Surg 1990; 116 (9): 1055–1060
52. Ginsberg, LE DeMonte, F. Imaging of perineural tumor spread from palatal carcinoma. AJNR Am J Neuroradiol. 1998; 19 (8): 1417–1422
53. Barakos, JA Dillon, WP Chew, WM. Orbit, skull base, and pharynx: contrast-enhanced fat suppression MR imaging. Radiology 1991; 179 (1): 191–198
54. Cruveilhier, J. Maladies des nerfs. In: Anatomie pathologique du corps humain (2nd ed.) Paris: JB Bailliére; 1835: 33, plate 34
55. Ernst, P. Ueber das Wachstum und die Verbeitung bösartiger Geshwü lste, insbesondere des Krebes in den Lymphbahnen der Nerven. Beitrr Path Anat Supple 1905; 7: 29–51
56. Rodin, AE Larson, DL Roberts, DK. Nature of the perineural space invaded by prostatic carcinoma. Cancer. 1967; 20 (10): 1772–1779
57. Anderson, TD Feldman, M Weber, RS Ziober, AF Ziober, BL. Tumor deposition of laminin-5 and the relationship with perineural invasion. Laryngoscope 2001; 111 (12): 2140–2143
58. McLaughlin, RB Jr Montone, KT Wall, SJ, et al. Nerve cell adhesion molecule expression in squamous cell carcinoma of the head and neck: a predictor of propensity toward perineural spread. Laryngoscope 1999; 109 (5): 821–826
59. Frierson, HF Jr El-Naggar, AK Welsh, JB, et al. Large-scale molecular analysis identifies genes with altered expression in salivary adenoid cystic carcinoma. Am J Pathol. 2002; 161 (4): 1315–1323
60. Conley, JJ Casler, JD. Data and statistics. In: Conley, JJ Casler, JD Perzin, K, eds. Adenoid Cystic Cancer of the Head and Neck. Stuttgart and New York: Georg Thieme Verlag Medical Publishers; 1991
61. Spiro, RH Huvos, AG. Stage means more than grade in adenoid cystic carcinoma. Am J Surg 1992; 164 (6): 623–628
61. van der Wal, JE Becking, AG Snow, GB van der Waal, I. Distant metastases of adenoid cystic carcinoma of the salivary glands and the value of diagnostic examinations during follow-up. Head Neck 2002; 24 (8): 779–783
63. Mazer, TM Robbins, KT McMurtrey, MJ Byers, RM. Resection of pulmonary metastases from squamous carcinoma of the head and neck. Am J Surg 1988; 156 (4): 238–242
64. Spiro, RH. Distant metastasis in adenoid cystic carcinoma of salivary origin. Am J Surg 1997; 174 (5): 495–498
65. Levin, JM Robinson, DW Lin, F. Acinic cell carcinoma: collective review, including bilateral cases. Arch Surg 1975; 110 (1): 64–68
66. Sakai, O Nakashima, N Takata, Y Furuse, M. Acinic cell carcinoma of the parotid gland: CT and MRI. Neuroradiology. 1996; 38 (7): 675–679
67. Batsakis, JG Luna, MA El-Naggar, AK. Histopathologic grading of salivary gland neoplasms: II. Acinic cell carcinomas. Ann Otol Rhinol Laryngol 1990; 99 (11): 929–933
68. Lewis, JE Olsen, KD Weiland, LH. Acinic cell carcinoma: clinicopathologic review. Cancer 1991; 67 (1): 172–179
69. Timon, CI Dardick, I Panzarella, T Thomas, J Ellis, G Gullane, P. Clinicopathological predictors of recurrence for acinic cell carcinoma. Clin Otolaryngol 1995; 20 (5): 396–401
70. El-Naggar, AK Abdul-Karim, FW Hurr, K Callender, D Luna, MA Batsakis, JG. Genetic alterations in acinic cell carcinoma of the parotid gland determined by microsatellite analysis. Cancer Genet Cytogenet 1998; 102 (1): 19–24
71. Spiro, RH Huvos, AG Strong, EW. Acinic cell carcinoma of salivary origin: a clinicopathologic study of 67 cases. Cancer 1978; 41 (3): 924–935
72. Spafford, PD Mintz, DR Hay, J. Acinic cell carcinoma of the parotid gland: review and management. J Otolaryngol 1991; 20 (4): 262–266
73. Lo, AK Topf, JS Jackson, IT Silberberg, B. Minor salivary gland basal cell adenocarcinoma of the palate. J Oral Maxillofac Surg 1992; 50 (5): 531–534
74. Warrick, PD Irish, JC Mancer, K Dardick, I Pynn, BR Gullane, P. Basal cell adenocarcinoma: a rare malignancy of the salivary glands. J Otolaryngol 2000; 29 (2): 102–109
75. Jayakrishnan, A Elmalah, I Hussain, K Odell, EW. Basal cell adenocarcinoma in minor salivary glands. Histopathology 2003; 42 (6): 610–614
76. Zimmerman, K. [Summary of information on epithelial glands]. Arch Mikroskopische Anatomie. 1898; 9: 163–168
77. Batsakis, JG Kraemer, B Sciubba, JJ. The pathology of head and neck tumors: the myoepithelial cell and its participation in salivary gland neoplasia, Part 17. Head Neck Surg 1983; 5 (3): 222–233
78. Di Palma, S Guzzo, M. Malignant myoepithelioma of salivary glands: clinicopathological features of ten cases. Virchows Arch A Pathol Anat Histopathol 1993; 423 (5): 389–396
79. Savera, AT Sloman, A Huvos, AG Klimstra, DS. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 25 patients. Am J Surg Pathol 2000; 24 (6): 761–774
80. Manolidis, S Donald, PJ Gandour-Edwards, R. Myoepithelial carcinoma: a report of two cases and a review of the literature. Otorhinolaryngol Nova 1997–98; 7: 220–226
81. Ellis, GL Auclair, PL Gnepp, DR. Surgical Pathology of the Salivary Glands. Philadelphia: WB Saunders; 1991
82. Wang, B Brandwein, M Gordon, R Robinson, R Urken, M Zarbo, RJ. Primary salivary clear cell tumors—a diagnostic approach: a clinicopathologic and immunohistochemical study of 20 patients with clear cell carcinoma, clear cell myoepithelial carcinoma, and epithelial-myoepithelial carcinoma. Arch Pathol Lab Med 2002; 126 (6): 676–685
83. Luna, MA Ordonez, NG Mackay, B Batsakis, JG Guillamondegui, O. Salivary epithelial-myoepithelial carcinomas of intercalated ducts: a clinical, electron microscopic, and immunocytochemical study. Oral Surg Oral Med Oral Pathol. 1985; 59 (5): 482–490
84. Donath, K Seifert, G Schmitz, R. Diagnosis and ultrastructure of the tubular carcinoma of salivary gland ducts: epithelial-myoepithelial carcinoma of the intercalated ducts [in German]. Virchows Arch A Pathol Pathol Anat 1972; 356 (1): 16–31
85. Corio, RL Sciubba, JJ Brannon, RB Batsakis, JG. Epithelialmyoepithelial carcinoma of intercalated duct origin: a clinicopathologic and ultrastructural assessment of sixteen cases. Oral Surg Oral Med Oral Pathol 1982; 53 (3): 280–287
86. Simpson, RH Clarke, TJ Sarsfield, PT Gluckman, PG. Epithelialmyoepithelial carcinoma of salivary glands. J Clin Pathol 1991; 44 (5): 419–423
87. Fonseca, I Soares, J. Epithelial-myoepithelial carcinomas. Am J Clin Pathol 1994; 101 (2): 242
88. Fonseca, I Soares, J. Epithelial-myoepithelial carcinoma of the salivary glands: a study of 22 cases. Virchows Arch A Pathol Anat Histopathol 1993; 422 (5): 389–396
89. Kleinsasser, O Klein, HJ Hubner, G. Salivary duct carcinoma: a group of salivary gland tumors analogous to mammary duct carcinoma [in German]. Arch Klin Exp Ohren Nasen Kehlkopfheilkd 1968; 192 (1): 100–105
90. Chen, KT Hafez, GR. Infiltrating salivary duct carcinoma: a clinicopathologic study of five cases. Arch Otolaryngol 1981; 107 (1): 37–39
91. Hui, KK Batsakis, JG Luna, MA Mackay, B Byers, RM. Salivary duct adenocarcinoma: a high grade malignancy. J Laryngol Otol 1986; 100 (1): 105–114
92. Gnepp, DR Chen, JC Warren, C. Polymorphous low-grade adenocarcinoma of minor salivary gland: an immunohistochemical and clinicopathologic study. Am J Surg Pathol 1988; 12: 461–468
93. Kemp, BL Batsakis, JG El-Naggar, AK Kotliar, SN Luna, MA. Terminal duct adenocarcinomas of the parotid gland. J Laryngol Otol 1995; 109 (5): 466–468
94. Batsakis, JG Pinkston, GR Luna, MA Byers, RM Sciubba, JJ Tillery, GW. Adenocarcinomas of the oral cavity: a clinicopathologic study of terminal duct carcinomas. J Laryngol Otol. 1983; 97 (9): 825–835
95. Vincent, SD Hammond, HL Finkelstein, MW. Clinical and therapeutic features of polymorphous low-grade adenocarcinoma. Oral Surg Oral Med Oral Pathol 1994; 77 (1): 41–47
96. Batsakis, JG El-Naggar, AK Luna, MA. “Adenocarcinoma, not otherwise specified”: a diminishing group of salivary carcinomas. Ann Otol Rhinol Laryngol 1992; 101 (1): 102–104
97. Thackray, A Lucas, R. Tumors of the Major Salivary Glands (2nd series, fasc. 10). Washington, DC: Armed Forces Institute of Pathology; 1974: 107–117
98. Eneroth, CM Zetterberg, A. Malignancy in pleomorphic adenoma: a clinical and microspectrophotometric study. Acta Otolaryngol 1974; 77 (6): 426–432
99. Felix, A Rosa, J Fonseca, I Cidadao, A Soares, J. Laminin and collagen IV in pleomorphic adenoma and carcinoma ex-pleomorphic adenoma: an immunohistochemical study. Hum Pathol 1999; 30: 964–969
100. Angelov, A Klissarova, A Dikranian, K. Radioimmunological and immunohistochemical study of carcinoembryonic antigen in pleomorphic adenoma and mucoepidermoid carcinoma of the salivary glands. Gen Diagn Pathol 1996; 141: 229–234
101. Spiro, RH Huvos, AG Strong, EW. Malignant mixed tumor of salivary origin: a clinicopathologic study of 146 cases. Cancer 1977; 39: 388–396
102. Tortoledo, ME Luna, MA Batsakis, JG. Carcinomas ex-pleomorphic adenoma and malignant mixed tumors: histomorphologic indexes. Arch Otolaryngol 1984; 110: 172–176
103. Carson, HJ Tojo, DP Chow, JM Hammadeh, R Raslan, WF. Carcinosarcoma of salivary glands with unusual stromal components: report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995; 79 (6): 738–746
104. Raja, V China, C Masaki, KH Nakano, G. Unusual presentations of uncommon tumors: Case 1. Benign metastasizing pleomorphic adenoma. J Clin Oncol 2002; 20 (9): 2400–2403
105. Perrin, TL. Mixed tumor of the parotid with metastases. Arch Pathol Lab Med 1942; 33: 930–934
106. McFarland, J. The mysterious mixed tumors of the salivary glands. Surg Gynecol Obstet 1943; 76: 23–34
107. McDowell, F. The rare, inexorable, lethal “benign mixed tumor” of the parotid. Plast Reconstr Surg 1975; 55 (2): 214–215
108. Klijanienko, J El-Naggar, AK Servois, V Rodriguez, J Validire, P Vielh, P. Clinically aggressive metastasizing pleomorphic adenoma: report of two cases. Head Neck. 1997; 19 (7): 629–633
109. Conley, J. The rare, inexorable, lethal benign mixed tumor of the parotid [letter]. Plast Reconstr Surg 1975; 56 (2): 205
110. Donovan, DT Conley, JJ. Capsular significance in parotid tumor surgery: reality and myths of lateral lobectomy. Laryngoscope 1984; 94 (3): 324–329
111. Phillips, PP Olsen, KD. Recurrent pleomorphic adenoma of the parotid gland: report of 126 cases and a review of the literature. Ann Otol Rhinol Laryngol 1995; 104 (2): 100–104
112. Laccourreye, H Laccourreye, O Cauchois, R Jouffre, V Menard, M Brasnu, D. Total conservative parotidectomy for primary benign pleomorphic adenoma of the parotid gland: a 25-year experience with 229 patients. Laryngoscope 1994; 104 (12): 1487–1494
113. El-Naggar, A Batsakis, JG Kessler, S. Benign metastatic mixed tumours or unrecognized salivary carcinomas? J Laryngol Otol 1988; 102 (9): 810–812
114. Greene, FL Page, DL Fleming, ID, et al. AJCC Cancer Staging Manual (6th ed). New York: Springer; 2002
115. Auclair, PL Langloss, JM Weiss, SW Corio, RL. Sarcomas and sarcomatiod neoplasms of the major salivary gland regions: a clinicopathologic and immunohistochemical study of 67 cases and review of the literature. Cancer 1986; 58: 1305–1315
116. Luna, MA Tortoledo, ME Ordonez, NG, et al. Primary sarcomas of the major salivary glands. Arch Otolaryngol Head Neck Surg 1991; 117: 302–306
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


