22
Local Complications of Dental Local Anesthesia
Local complications caused by or associated with dental local anesthesia can be classified according to onset (Laskin 1984; Vega 1998): (i) as immediate or accidental complications, when they appear during treatment in the office, or (ii) as late complications, or simply complications, when they appear after the patient has left the office.
Local complications are more frequent than general complications, although, fortunately, the vast majority resolve spontaneously without severe problems for the patient and without leaving sequelae (Laskin 1984). Table 22.1 shows these complications, their association with onset (immediate or late), and their severity.
In 1997 (Vega 1998) and during 2000–2010 (Perea‐Perez et al. 2014), 4% of adverse effects (local or general) recorded in Spain were associated with dental local anesthesia.
Persistent Post‐injection Pain
Disappearance of local anesthesia effect is sometimes followed by slight discomfort at the injection site, which is usually of little clinical relevance (Jorkjend and Skoglund 1999); however, persistent moderate‐to‐severe pain may be present and may last several days.
Persistent post‐injection pain is unusual in maxillary buccal infiltration and affects approximately 1% of cases of persistent pain (Table 22.2). This is natural, given the low degree of trauma involved in this technique at this level (Kennedy et al. 2001; Evans et al. 2008), and the pain disappears spontaneously within 3 days (Evans et al. 2008). Post‐injection pain is much more frequent in mandibular block and is thought to affect 5% of cases (Table 22.2), possibly because it is a much more traumatic technique. This complication usually resolves spontaneously within 3–4 days (Dunbar et al. 1996; Ridenour et al. 2001; Mikesell et al. 2005).
Self‐inflicted Injury
Anesthesia of the soft tissue persists after the dental treatment session, and some patients (mainly young children and developmentally disabled patients) may bite and injure their lips, jugal mucosa, and tongue.
The frequency of these lesions was unknown until recently, not because they were uncommon, but because of the few problems they generally cause. Pediatric dentists estimate their frequency to be 5% or slightly greater (College et al. 2000; Ram and Amir 2006), and studies based on case series agree, reporting the frequency to be around 7% (Table 22.3). However, this percentage only refers to the most severe cases, given that clinical studies focusing on self‐inflicted injury report a frequency of 13% (College et al. 2000; Adewuni et al. 2008), possibly because they include small lesions such as redness or swelling that are noticed by the dentist but not by parents (College et al. 2000). The lesions affect the lips (13%), buccal mucosa (10%) (cheeks), and tongue (6%) (Adewuni et al. 2008).
Once soft tissue anesthesia resolves, red and inflamed lesions appear on the affected tissues and, in the more severe cases, may become ulcerated and painful. The lesions heal spontaneously in less than 2 weeks. We have already seen that the cause is accidental biting of the anesthetized tissues. Occasional cases of burning by cigarette smoke have been recorded in adults. The associated factors are as follows:
- Age. Smaller children are more likely to experience self‐inflicted injury because of their age (College et al. 2000; Adewuni et al. 2008). The same applies to patients with intellectual disability.
- The duration of anesthesia in soft tissues. The longer the duration, the greater the frequency of self‐inflicted injuries (Adewuni et al. 2008).
Table 22.1 Local complications of dental local anesthesia: severity and onset.
Complication Severity Onset Mild Severe Immediate Late - Persistent pain
+ + - Self‐inflicted injury
+ + - Facial blanching
+ + - Delay cutaneous lesion
+ + - Facial hematoma
+ + + - Nerve lesions
+ + + + - Trismus
+ + + + - Facial palsy
+ + + + - Ocular complications
+ + + + - Infections
+ + - Mucosal ulceration
+ + + - Breakage of needles
+ + - Breakage of cartridges
+ + + - Aural complications
+ + + Table 22.2 Moderate to severe pain after maxillary buccal infiltration and mandibular block.
Reference Sample size Persistent pain Maxillary infiltration Kennedy et al. (2001) 120 0% Evans et al. (2008) 80 0% Hersh et al. (2008) 122 2.5% 0.83% ≈ 1% Mandibular block Krafft and Hickel (1994) 12 104 0.32% Dunbar et al. (1996) 40 5% Reitz et al. (1998) 38 0% Ridenour et al. (2001) 30 14% Mikesell et al. (2005) 57 5% Mikesell et al. (2005) 57 21% Goodman et al. (2006) 46 7% Hersh et al. (2008) 122 5% Willett et al. (2008) 25 8% 7% ≈ 5% - Bilateral mandibular block. Surprisingly, bilateral block has a lower incidence of self‐inflicted lesions than unilateral block, possibly for two reasons (College et al. 2000):
- Bilateral mandibular block is used in more extensive and longer treatments, therefore duration of soft tissue anesthesia is shorter when the child leaves the dentist’s office.
Table 22.3 Percentage of lesions resulting from biting after dental local anesthesia.
Reference Age (years) Lesions College et al. (2000) 2–18 13% Malamed et al. (2001) <13 0.07% Ram and Amir (2006) 5–13 5% Adewuni et al. (2008) 2–14 13% Peñarrocha‐Oltra et al. (2012) 11–55 4% Hersh et al. (2017) 2–5 2% 6.2% ≈ 5% - The sensation of symmetrical paresthesia on both sides means that the patient is less likely to explore and bite the tissue.
- Bilateral mandibular block is used in more extensive and longer treatments, therefore duration of soft tissue anesthesia is shorter when the child leaves the dentist’s office.
The measures that dentists can take to prevent, or at least reduce, the probability of these lesions in children are as follows:
- Explain to patients (especially children and their caregivers) that they cannot chew or have very hot drinks while the lips continue to feel numb (tingling sensation, numbness, dullness, swollen or fat lip).
- Adults should be advised not to smoke.
- Some patients claim that they can chew with the other (nonanesthetized) side, although it must be stressed that both sides tend to be used unconsciously when chewing and that chewing may be more difficult than the patients perceive it to be.
- This must be emphasized to both children and parents.
- Place a cotton roll between the lips and cheek. This is a commonly utilized practice in pediatric dentistry; the cotton roll is left in place for as long as the soft tissue anesthesia persists, reminding the patient not to chew their lips or cheeks.
- Phentolamine (OraVerse®) could prove very useful in the areas where the anesthetic solution has been injected to reduce numbness in the soft tissue after treatment (Tavares et al. 2008; Zurfluh et al. 2015; Hersh et al. 2017) (Chapter 6).
Facial Blanching
Although the real frequency of this condition is unknown, it is thought to be frequent and has been reported to account for 1% of all cases of numbness caused by dental local anesthetic (Lustig and Zusman 1999).
Blanching (ischemic paling) is characterized by blood loss during the injection or immediately after. It affects the skin of the face (cheek) on the maxilla and disappears spontaneously after 10–30 minutes without sequelae (Kronman and Giunta 1987; Heasman and Reid 1995; Aravena et al. 2016).
Anesthetic Techniques Involved
- Mandibular block with the conventional or direct technique (Kronman and Giunta 1987; Heasman and Reid 1995; Webber et al. 2001; Uckan et al. 2006; Paul et al. 2009; Aravena et al. 2016), the Gow‐Gates technique (Dryden 1993) or the Laguardia–Akinosi technique (Donkor et al. 1990).
- In the maxillary arch, the techniques involved are buccal infiltration, posterior superior alveolar nerve block, the high tuberosity technique (Kronman and Giunta 1987; Lustig and Zusman 1999), and the transpalatal (greater palatine canal) approach (Mercuri 1979).
Clinical Manifestations
Blanching appears on the same side of the face as the injection, with the following:
- Facial blanching, which is characterized by the following:
- Onset on the skin of the face below the lower eyelid (infraorbital), malar or zygomatic region, side of the nose, and above the nasolabial fold (cheek). It manifests as a continuous white patch (Figure 22.1) or occasionally two patches (Kronman and Giunta 1987). Blanching is easily observed in persons whose skin is not black (Mercuri 1979) and disappears spontaneously after 10–30 minutes (Kronman and Giunta 1987; Heasman and Reid 1995; Paul et al. 2009; Aravena et al. 2016; Kumaresan et al. 2018).
- The patch can sometimes extend intraorally to the maxillary gingiva (Heasman and Reid 1995; Aravena et al. 2016), hard palate (Dryden 1993; Heasman and Reid 1995; Aravena et al. 2016; Kumaresan et al. 2018), or even to the lower lip (Webber et al. 2001).
- An asymptomatic sign (only observed by the dentist), although the patient may initially feel numbness, itching, a burning sensation, or even shooting pain, in the affected area (Mercuri 1979; Webber et al. 2001; Uckan et al. 2006; Paul et al. 2009; Aravena et al. 2016; Kumaresan et al. 2018).
- While rare, ocular complications (see section “Ocular Complications”) of the have been described (Dryden 1993; Webber et al. 2001; Chiappelli and Cajulis 2002; Uckan et al. 2006) and include the following:
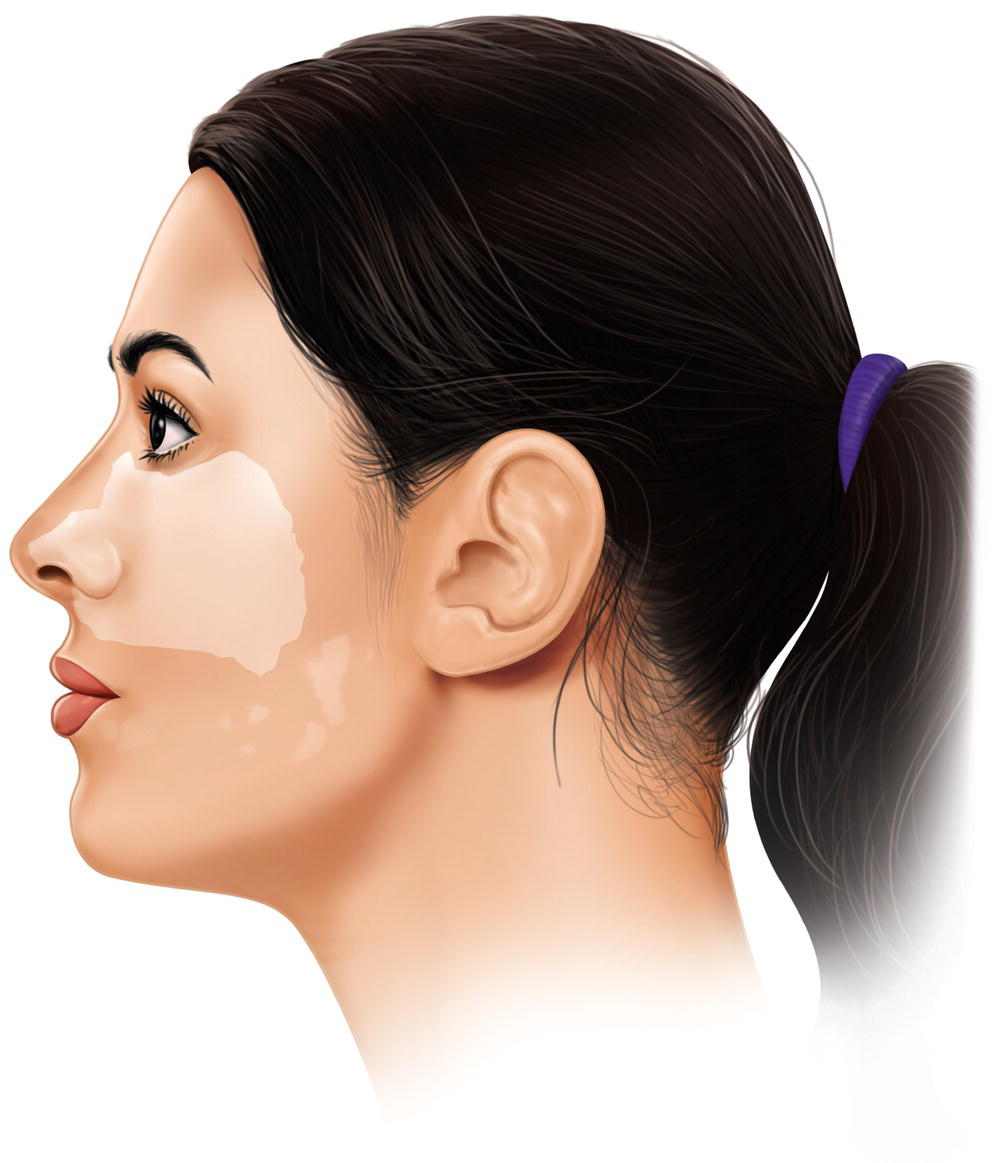
Figure 22.1 Area affected by facial blanching.
- Double vision (diplopia).
- Sensation of numbness, burning, or even pain in the eye and periorbital area.
- Drooping of the upper eyelid (ptosis).
- Ischemic paling may extend to the eyelids and forehead (Webber et al. 2001; Uckan et al. 2006).
In these cases, ocular manifestations are the main event, with blanching no more than an accompanying sign.
Causes and Pathophysiology
Proposed Causes
- Irritation of sympathetic fibers surrounded the arteries (perivascular sympathetic plexus) by the tip of the needle at the injection site can cause reflex vasoconstriction along the terminal branches, in this case at the level of the skin (this may occur internally, although we do not see it). Since aspiration is negative in most cases, this seems to be the most common explanation (Laskin 1984; Kronman and Giunta 1987; Lustig and Zusman 1999).
- Epinephrine from the local intravascular anesthetic injected is transported to the terminal peripheral branches of the skin of the face (Kumaresan et al. 2018).
Pathophysiology
Vasoconstriction follows the path of the superior alveolar arteries (in injections into the superior arch) or of the lower alveolar arteries (in mandibular block) to the maxillary artery, from where it will reach the infraorbital artery, whose branches perfuse the skin (zygoma, infraorbital border, wing of the nose). One variant is from the maxillary artery to the greater palatine artery (palate).
Injecting directly into the maxillary artery (not from the alveolar arteries) is also possible (Kronman and Giunta 1987; Heasman and Reid 1995). It is important to remember the anatomical variations of the maxillary artery, which runs superficially and laterally to the lateral (external) pterygoid muscle at this level (Pretterklieber et al. 1991). The maxillary artery varies widely in diameter (2–6 mm) (Biermann 1943) and often descends to the mandibular foramen (Lacouture et al. 1983) (Figure 3.13, Chapter 13).
Another possibility is the retrograde flow of the anesthesia, and especially the vasoconstrictor, from the alveolar arteries to the maxillary artery. This option is less probable (Heasman and Reid 1995).
Localized Late‐onset Skin Lesion
The frequency of this type of lesion, which affects the skin of the lips, is unknown, although it is thought to be exceptional, given that very few cases have been reported. It is noteworthy that most occur after mandibular block in children aged 7–10 years (Table 22.4).
Clinical Manifestations
The affected skin depends on the region where the anesthesia is injected. Thus, in cases of mandibular block, the skin of the lower lip is affected above the chin or adjacent to the commissure; the skin of the upper lip is affected in maxillary infiltrations.
Clinical manifestations appear during the first hours (30 minutes to 3 hours) after administration of the anesthetic (rarely before 3 days) (Table 22.4) and are characterized by the appearance of a reddish patch (erythematous macule), which is usually accompanied by itching (pruritus) or a burning sensation. Occasionally, it first manifests as a pale patch that progresses to an erythematous macule within a few hours (Torrente‐Castells et al. 2008).
During the following days or weeks, the lesion progresses to necrosis of the skin with formation of a crust that leaves a pigmented or hypopigmented area or simply a scar on healing. Sensory alterations on the chin are a potential sequela (Krüger and Nehse 1991).
Causes and Pathophysiology
The causes are not well known, although two possible mechanisms have been posited (Curley and Baxter 1987):
Ischemic Necrosis Due to Vasospasm
Vasoconstriction results from needle‐induced irritation of the sympathetic fibers surrounding the arterial wall, thus leading to vasoconstriction along the terminal branches, in this case at the level of the skin. It may also be caused by exogenous epinephrine injected intravascularly that is transported toward the terminal peripheral branches of the skin of the face.
Table 22.4 Characteristics of cases of localized delayed skin lesion
| Reference | Age/sex | Technique | Skin affected | Anesthetic | Time to onset |
|---|---|---|---|---|---|
| ml/LAS | |||||
| Lederman et al. (1980) | 7/♀ | MB left | Lower lip Chin |
1.8/L‐100 | 30 min |
| 8/♀ | MB left | Lower lip Chin |
<1.8/L‐100 | 1 h | |
| 7/♀ | MB left | Lower lip near commissure | 1.8/L‐100 | 45 min | |
| 9/♀ | MB left | Lower lip near commissure | −/L‐100 | 2 h | |
| Curley and Baxter (1987) | 7/♀ | InfP left | Upper lip | 2.2/L‐50‐50 | Hours |
| Krüger and Nehse (1991) | 33/♀ | MB left + inf mental | Lower lip Chin |
5.4/A‐100 | 3 days |
| Torrente‐Castells et al. (2008) | 10/♀ | MB left + inf mental | Lower lip Chin |
1.8/A‐100 | 3 h |
MB, mandibular block; inf mental, infiltration in mental nerve; InfP, buccal infiltration in posterior upper arch (molars); ml, injected milliliters; LAS, local anesthetic solution: L‐100, lidocaine 2% with epinephrine 1:100 000; A‐100, articaine 4% with epinephrine 1:100 000; L‐50‐50, lidocaine with epinephrine 1:50 000 and with norepinephrine 1:50 000.
In mandibular block, the path covered by the vasoconstrictor effect runs from the inferior alveolar artery (branch of the maxillary artery) to its mental branch and from here by anastomosis (Kawai et al. 2006) with the submandibular and inferior labial arteries (both branches of the facial artery), leading to vasoconstriction of the vessels of the skin of the chin (Torrente‐Castells et al. 2008). Direct injection into the area of the mental nerve is an aggravating factor, leading to vasospasm of the arteries that supply the skin of the chin and the intraoral mucosa in the region of the lower canine and first mandibular premolar (Krüger and Nehse 1991; Torrente‐Castells et al. 2008).
Type III Allergic Reaction
A type III allergic reaction, or immune complex–mediated reaction (e.g. Arthus reaction or serum sickness), is an antigen–antibody reaction in the walls of the blood vessels that leads to acute vasculitis with tissue necrosis. This reaction manifests locally within a few hours. It very rarely occurs with local anesthetics (Lederman et al. 1980).
In cases of type III allergic reaction, skin allergy tests usually yield negative results (Lederman et al. 1980; Curley and Baxter 1987), although when the same drug is applied at the same site, the late skin eruption re‐occurs. However, when another solution is used, the reaction does not appear (Lederman et al. 1980).
Facial Hematomas
A hematoma is caused by extravasation of blood from a vessel to the surrounding tissue as a result of needle injury (Kuster and Udin 1984). If the vessel is an artery, blood accumulates quickly; if it is a vein, blood accumulates slowly (Laskin 1984). In all cases, bleeding is self‐limiting because of pressure from the surrounding tissue.
Many techniques can lead to hematoma, for example mandibular block, although given that the vessels are very deep, the hematoma is not clinically visible (Kuster and Udin 1984). Even so, small hematomas are common on the oral mucosa as a result of techniques involving maxillary infiltration into the lateral incisors and first molars: hematoma has been estimated to appear in 1% of cases (Evans et al. 2008), although with the minimum volume/minimum injection time technique, this frequency is multiplied since several injections are made at the same site.
In this section, we will examine hematomas that appear on the skin of the face, but not the smaller yet common hematomas that appear on the mucosa.
Technical Factors Contributing to Hematomas
The techniques that most frequently lead to hematomas on the face are as follows:
- Injections into the area of the upper molars in buccal infiltration, posterior superior alveolar nerve block, and high tuberosity approaches (Bennett 1984; Laskin 1984; Roberts and Sowray 1987; Jastak et al. 1995; Malamed 2004). At this level, 0.5% of injections can lead to facial hematoma (Kuster and Udin 1984), especially when the needle is inserted higher and deeper, since it is easier to inject into a branch of the pterygoid venous plexus or the posterior superior alveolar artery (Harn et al. 2002).
- Block applied at the level of the foramina (infraorbital and mental) (Laskin 1984; Roberts and Sowray 1987; Joyce and Donnelly 1993; Jastak et al. 1995; Malamed 2004; Karkut et al. 2010). With some exceptions and specific cases such as those already discussed, these techniques are not recommended in current practice (Evers and Haegerstam 1981; Kleier et al. 1983; Haglund and Evers 1985; Joyce and Donnelly 1993).
Clinical Manifestations
- Swelling, which appears in the area of the upper molars (Kuster and Udin 1984) and can appear on the skin of the malar region or masseter. This swelling is very large and cosmetically undesirable because a large quantity of blood can accumulate in the infratemporal space, thus highlighting the resulting facial asymmetry (Malamed 2004). Swelling appears rapidly if the lesion affects an artery, such as a branch of the facial artery or a buccogingival branch of the posterior superior alveolar artery, which follows an irregular path along the maxillary tuberosity (Jastak et al. 1995; Harn et al. 2002; Malamed 2004), or slowly if the lesion affects a vein of the pterygoid venous plexus (Jastak et al. 1995; Malamed 2004).
- Skin discoloration, also known as ecchymosis (Bennett 1984; Kuster and Udin 1984; Roberts and Sowray 1987; Malamed 2004), progresses downwards and forwards along the muscle planes of the cheek until it reabsorbs spontaneously after 10–15 days (Malamed 2004).
- Other occasional manifestations include the following:
Management by the Dentist
- The patient should be advised that the hematoma reabsorbs within 10–15 days (Bennett 1984; Malamed 2004). Often no interventions are recommended and invasive measures such as drainage are contraindicated (Bennett 1984; Roberts and Sowray 1987; Jastak et al. 1995; Malamed 2004).
- If the swelling is detected early, the dentist may attempt to control it by pressing on the affected area for 15 minutes (Kuster and Udin 1984; Laskin 1984) and/or applying ice to the skin for its vasoconstrictive effect (Kuster and Udin 1984; Jastak et al. 1995; Malamed 2004). In the case of a hematoma affecting the region of the upper molars, it is difficult to apply direct pressure, therefore a finger should be inserted directly into the mouth and pressure applied at the bottom of the vestibular surface of the upper molars, whereas on the outside pressure can be applied to the skin of the malar region (Malamed 2004).
- As an option, some authors recommend that the patient apply heat to the affected area 24 hours after the procedure to aid reabsorption of the hematoma (Laskin 1984; Jastak et al. 1995; Malamed 2004). Heat causes vasodilation, favors withdrawal of the extravasated blood, and has an analgesic effect.
- Some authors suggest reassessing the lesion at 48 hours to determine whether an infection has developed (very unusual) and prescribe antibiotics (Laskin 1984).
Nerve Lesions
This section includes nerve lesions caused by injection of dental local anesthesia. Thus, we can mention the following:
- Electric shock sensation on insertion of the needle.
- Long‐term paresthesia caused by persistent neuropathy.
- Alterations of the sense of taste caused by injury to the chorda tympani.
- Hoarseness by block of recurrent laryngeal nerve.
Anatomical Lesions
In 1943, Seddon described three basic types of lesion of the peripheral nerves (Seddon 1943):
- Neurapraxia. When there is no axonal degeneration, although the axons are intact, they do not conduct electrochemical impulses. There is no loss of axonal continuity. In these cases, recovery is spontaneous (10 days to 3 weeks).
- Axonotmesis. When there is axonal degeneration. There is no anatomical damage to the nerve, and although the axon is split, the nerve stem remains intact thanks to the supporting connective tissue. Recovery is spontaneous (6–8 weeks or 2–6 months).
- Neurotmesis. When there is axonal degeneration and anatomical damage to the nerve and therefore complete rupture of the nerve stem. The resulting lesion is permanent and a scar neuroma may form as a result of interference in neuronal regeneration.
General Causes
- Needle injury (physical effect). The tip of the needle, especially if barbed outwards (typical in mandibular block after pressing the needle against the bone) (Stacy et al. 1994), can directly injure the nerve stem and lead to intraneural hemorrhage (Haas and Lennon 1995; Pogrel and Thamby 2000) (Figure 22.2), which in turn could increase pressure on the nerve fibers, thus altering metabolism and nerve function (Haas and Lennon 1995). The situation is aggravated by the subsequent intra‐ and extraneural fibrosis (Pogrel and Thamby 2000).
Repeating the number of injections, especially in mandibular block, increases the risk of this type of lesion (Pogrel et al. 1995; Pogrel and Thamby 2000; Hillerup and Jensen 2006). In some series, 35% of patients report having received more than two injections at the same site (Hillerup and Jensen 2006).
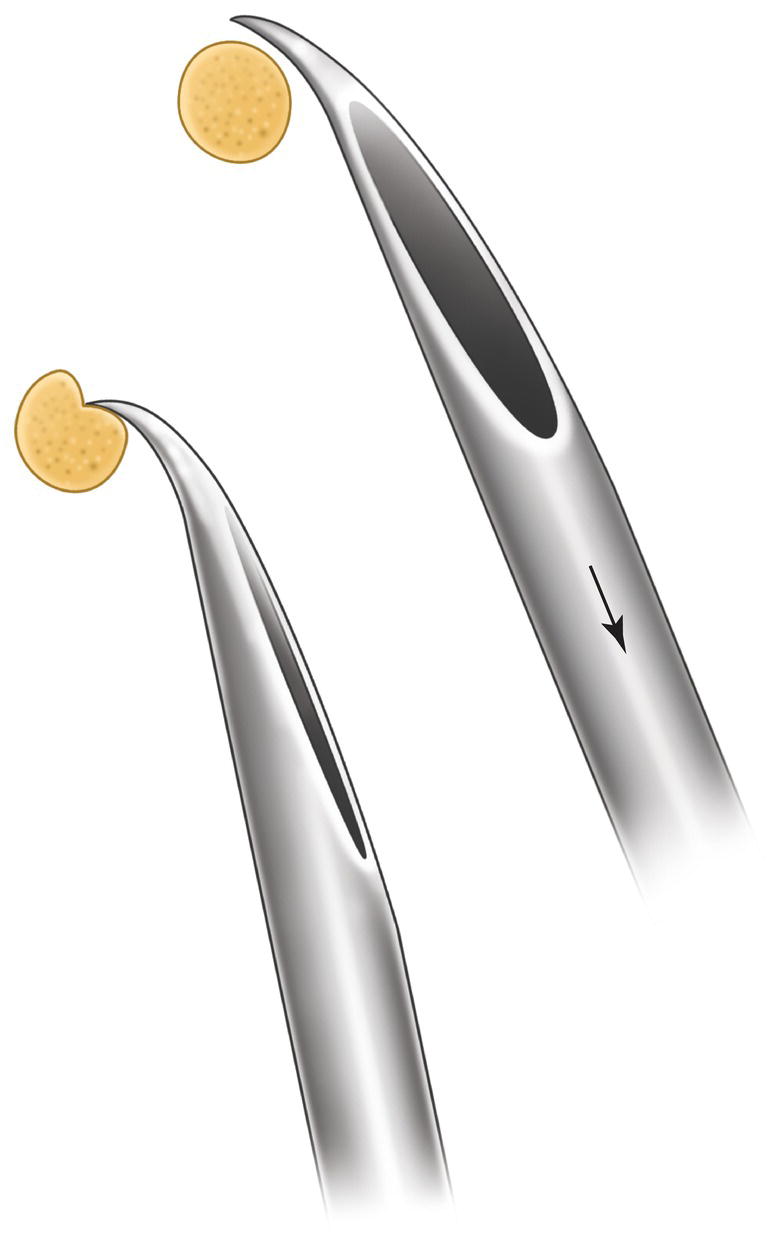
Figure 22.2 Nerve injury caused by a needle barbed outward.
Source: Redrawn from Stacy et al. (1994).
Note: It is interesting that, in five cases involving exploratory surgery, no evidence of needle‐induced microtrauma was observed, although the area was slightly pale and there were adhesions around the nerve (Pogrel and Thamby 2000).
- Neurotoxicity caused by contamination of anesthetic solution (chemical effect). For some time, professionals had a bad habit of submerging the local anesthesia cartridges in surface disinfectants, often alcohol, with the result that the disinfectant entered the cartridge through the diaphragm or the rubber plunger (made from semipermeable membranes), thus making the injection more painful and irritating the tissues and, more importantly, the nerve (Shannon and Feller 1972; Shannon and Wescott 1974).
- Neurotoxicity caused by the individual components of the local anesthesia solution (chemical effect). Experiments with animals (Lundy et al. 1933; Tui et al. 1944; Skou 1954; Fink and Kish 1976; Myers et al. 1986; Kalichman et al. 1993) have demonstrated the neurotoxic effect of local anesthetics, especially when these are used at high concentrations. Clinical studies have also demonstrated this adverse effect in medical practice (Rigler et al. 1991) and in dental practice (Nickel 1990; Haas and Lennon 1995; Miller and Haas 2000; Van Eeden and Patel 2002; Hillerup and Jensen 2006; Garisto et al. 2010), especially when high concentrations are administered, as is the case with lidocaine 5% (Rigler et al. 1991), articaine 4% (Haas and Lennon 1995; Miller and Haas 2000; Van Eeden and Patel 2002; Hillerup and Jensen 2006; Hillerup et al. 2011; Garisto et al. 2010), and prilocaine 4% (Haas and Lennon 1995; Miller and Haas 2000; Garisto et al. 2010).
Immediate Electric Shock Sensation
Immediate electric shock sensation (cramp) is mainly felt in mandibular block. When the needle is inserted, the patient experiences a sudden and short electric shock sensation, which is like an intense burning sensation along the nerve that has been touched by the tip of the needle (lingual nerve or inferior alveolar nerve). The sensation lasts a second but is very unpleasant for the patient, although it has the advantage of producing deep and quick anesthesia with a small amount of anesthetic solution.
The frequency of electric shock sensation in mandibular block is around 3% (Table 22.5), and this is more common in the lingual nerve than in the inferior alveolar nerve (ratio 4:1). In the case of the lingual nerve, the area affected by the sensation is the tongue on the side the anesthetic is injected; in the case of the inferior alveolar nerve, the area affected is the half of the lower lip on the side which the anesthetic is injected.
Table 22.5 Percentage of cases of electrical shock sensation after mandibular block and recovery.
| Reference | Sample size (cases/total) | Nerves affected | Electric shock | Total recovery |
|---|---|---|---|---|
| Harn and Durham (1990) | 347/9587 | Lingual | 3.6% | 85% |
| Krafft and Hickel (1994) | 856/12 104 | Lingual | 7% | 98% |
| Lustig and Zusman (1999) | 40/731 | Lingual and inferior alveolar | 5.5% | 100% |
| Pogrel and Thamby (2000) | 1/80 | Lingual | 1.3% | — |
| Pogrel and Thamby (2000) | 1/320 | Inferior alveolar | 0.3% | — |
| Nooh and Abdullah (2010) | 2/5000 | Lingual | 0.04% | 100% |
| Morris et al. (2010)a | 2/44 | Lingual | 4.5%a | — |
| Average | 3.2% | 95.7% | ||
| Rounded average | 3% | 95% |
a Cadaver, contact the needle with the nerve.
When faced with these situations in the office, we advise the following:
- Show the patient that you are aware how unpleasant the sensation is and state that this happens because the anesthetic was injected immediately above the nerve, when the normal approach is to inject it to the side.
- Tell the patient that the discomfort has the advantage that the anesthetic effect is quicker and stronger.
- In cases where this accident is repeated in the same patient, we advise against using mandibular block because it indicates a possible anatomical abnormality and repeated injury could carry a risk of long‐term paresthesia. As alternatives, we propose the use of another mandibular block technique such as Gow‐Gates (Chapter 16) or infiltrative techniques accompanied by intraligamentary or intraosseous approaches (Chapter 18).
Electric Shock Sensation After the Transpalatal Approach
An interesting variation of this problem is in the transpalatal (greater palatine canal) approach, which in 1% of cases can lead to an intense sensation of electric discharge or burning in the palate on the side of the injection (Sved et al. 1992). This occurs because the needle is inserted into the greater palatine canal, which leads to the pterygopalatine fossa, where the maxillary division of the trigeminal nerve (CN V2) is located.
No permanent or long‐term lesions have been reported at this level, probably because the technique is rarely used.
Long‐Term Paresthesia
The frequency of long‐term paresthesia or nonsurgical neuropathy caused by dental local anesthesia is unknown (Pogrel and Thamby 2000; Hillerup and Jensen 2006); however, some authors provide estimates, although these vary widely, ranging from 1:5000 injections to 1:14 million injections (Table 22.6). Clinical experience tells us that cases of long‐term paresthesia are not often seen in clinical practice, therefore a frequency ranging from 1:5000 to 1:10 000 seems excessive. Furthermore, the study by Garisto et al. (2010), which reported a frequency of 1:14 million, recognizes that this is improbable and that the frequency may be even greater. We believe that the true value lies somewhere between the figures reported (Table 22.6), that is, a median of 1 : 100 000 injections.
Table 22.6 Frequency of long‐term paresthesia after mandibular block, as estimated by various authors
| Reference | Nerves affected | Estimated frequency | |
|---|---|---|---|
| Estimated number | Round number | ||
| Harn and Durham (1990) | Lingual | 1:4743 | 1:5000 |
| Krafft and Hickel (1994) | Lingual | 1:12 104 | 1:10 000 |
| Pogrel and Thamby (2000)a | Lingual and inferior alveolar | 1:26 762 | 1:25 000 |
| Sambrook and Goss (2011)b | Lingual and inferior alveolar | 1:48 956 | 1:50 000 |
| Pogrel and Thamby (2000)a | Lingual and inferior alveolar | 1:160 571 | 1:160 000 |
| Ehrenfeld et al. (1992) | Lingual and inferior alveolar | 1:200 000 | 1:200 000 |
| Haas and Lennon (1995) | Lingual and inferior alveolar | 1:785 000 | 1:800 000 |
| Garisto et al. (2010) | Lingual and inferior alveolar | 1:13 800 970 | 1:14 000 000 |
Data ordered by estimated frequency.
a Data from Pogrel and Thamby (2000) (two series of patients).
b Estimated 30% of mandibular block (data from Annex 1).
By far the most frequently involved technique is mandibular block (99%) (Table 22.7) and the most frequently involved nerves are the lingual nerve (70%, tongue involvement), the inferior alveolar nerve (20%, involvement of half of the lower lip), and both nerves (10%) (Table 22.7). The reason for more frequent involvement of the lingual nerve seems to be that in 33% of cases it is composed of a single bunch of nerve fibers (possibly one to eight), whereas the inferior alveolar nerve is composed of three to 14 bundles, therefore injury is offset by the remaining healthy bundles (Pogrel et al. 2003; Khoury et al. 2010). Also, the inferior alveolar nerve may be partially protected from oncoming the needles by a crest of thickened bone, which bulges anteriorly in the sulcus colli, and the protection of the lingula (Khoury Mihailidis et al. 2011). In contrast, the lingual nerve is quite bare, with no bony protection, exposing it to an increased risk of direct contact during needle insertion due to its anteromedial position (Khoury Mihailidis et al. 2011) (Figure 3.14, Chapter 3).
Causes of Long‐term Lesions
- Needle‐tip injury, given that in 40% of cases there is a history of electric shock sensation, although this data varies widely between authors (Table 22.7).
- Neurotoxicity of local anesthetic solutions, especially those administered at high concentrations. Thus, articaine 4% has a 9‐fold higher risk than average and a 22‐fold greater risk than solutions with lidocaine 2%, which is the standard local anesthetic (Table 22.8). The same is true of prilocaine 4%, which carries a 4‐fold greater risk than average and a 35‐fold greater risk than lidocaine 2% (Table 22.8). Therefore, some authors do not recommend these two solutions for mandibular block (Hillerup and Jensen 2006; Hillerup et al. 2011), especially since the standard solution of lidocaine 2% with epinephrine yields similar efficacy for inferior alveolar nerve (Annex 24), under the usual conditions of dental work.
Clinical Manifestations
- Paresthesia and dysesthesia. These are the main manifestations; they occur in the area innervated by the affected nerve (half of the tongue for the lingual nerve or half of the lower lip and the chin for the inferior alveolar nerve). The sensation is abnormal and generally unpleasant, and may involve heat, loss of feeling, tingling, numbness, burning sensation, prickling, and even pain. The most characteristic sensation of paresthesia is tingling or numbness (Girard 1979) and dysesthesia (abnormal sense of touch).
There are variations with respect to pain, such as more increased sensitivity to a stimulus (hyperalgesia), pain with normally painless stimuli (allodynia), or absence of pain with stimuli that are normally painful (analgesia).
Table 22.7 Long‐term paresthesia: most frequently involved techniques, most affected nerves, and history of electric shock sensation.
Basic study data Technique used Nerves affected History of electric shock Reference Origin Cases Sample size Years of study Mandibular block Other Lingual Inferior alveolar Both Proportion Percentage N % N % N % Gerlach et al. (1989) Germany 12 — 1985–1988 100 0 7 58 5 42 0 0 — — Harn and Durham (1990) United States 51 41 1985–1990 100 0 51 100 0 0 0 0 52/52 100 Ehrenfeld et al. (1992) Germany 9 8 1987–1991 100 0 8 89 1 11 0 0 4/9 44 Krafft and Hickel (1994) Germany 18 18 1987–1990 100 0 18 100 0 0 0 0 0/18 0 Haas and Lennon (1995) Canada 143 — 1973–1993 100 0 92 65 42 30 9 6 31/143 22 Pogrel and Thamby (2000) United States 93 83 1983–2000 100 0 57 69 18 22 9 11 47/83 57 Hillerup and Jensen (2006) Denmark 54 52 1997–2004 100 0 40 77 10 19 2 4 20/36 55 Alcaina et al. (2010) Spain 2 2 — 100 0 1 50 0 0 1 50 1/2 50 Garisto et al. (2010) United States 226 — 1997–2008 95a 5 170 89 14 7 7 4 18/191 10 Hillerup et al. (2011) Denmark 115 — 2001–2007 94 6 — — — — — — — — Sambrook and Goss (2011) Australia 8 8 2009 100 0 2 25 4 50 2 25 — — Average 99% 1% 72% 18% 10% 42% Rounded average 70% 20% 10% 40% a Garisto et al. (2010) also report 4% high tuberosity block and 1% mental block (in total 5% other techniques).
Table 22.8 Greater risk of long‐term paresthesia after mandibular block with articaine 4% and prilocaine 4% with respect to the general average risk and lidocaine 2%, which is the standard anesthetic.
Anesthetic Greater risk than Times greater Reference Articaine 4% General risk 3.6 Garisto et al. (2010) 5 Hillerup et al. (2011) 5 Miller and Haas (2000) 11 Legarth (2005) 20 Hillerup and Jensen (2006) Mean 9 Lidocaine 2% 6.3 Hillerup et al. (2011) 15a Haas and Lennon (1995) 45 Garisto et al. (2010) Mean 22 Prilocaine 4% General risk 0 Legarth (2005) 5 Miller and Haas (2000) 7.5 Garisto et al. (2010) Mean 4 Lidocaine 2% 0 Legarth (2005) 11a Haas and Lennon (1995) 90 Garisto et al. (2010) Mean 35 a Estimated based on data from 1993 with consumption of lidocaine forecast from 1973 and of articaine from 1983 and averaging the total number of years.
There are also variations with respect to stimuli in general, for example loss of sensitivity to stimuli (anesthesia), reduced sensitivity to stimuli (hypoesthesia), or increased sensitivity to stimuli (hyperesthesia).
- Altered sense of taste in 50% of cases in which the lingual nerve is involved, owing to its association with the chorda tympani nerve (Table 22.9). The alterations the patient perceives are as follows:
Table 22.9 Occurrence altered taste in patients with injuries to the lingual nerve.
Reference Sample size (cases/total) Percentage Ehrenfeld et al. (1992) 5/8 63% Haas and Lennon (1995) 22/101 22% Hillerup and Jensen (2006) 33/42 79% Garisto et al. (2010) 44/170 26% Average 48% ≈ 50% - Reduced perception of taste (hypogeusia) and, more rarely, absence of taste (ageusia) (Haas and Lennon 1995; Hillerup and Jensen 2006).
- Altered perception of taste with a burning sensation on the tongue, bitter taste, or bad taste (dysgeusia) (Haas and Lennon 1995; Hillerup and Jensen 2006).
Of clinical interest, there are no differences in involvement between the right and left sides (Harn and Durham 1990; Haas and Lennon 1995; Pogrel and Thamby 2000).
- These symptoms may very occasionally be accompanied by painful ulceration on the dorsum of the tongue, which usually resolves after a few weeks when the paresthesia disappears (Martis 1969), or late‐onset trismus (Smyth and Marley 2010).
Management by the Dentist
The recommendations are as follows:
- Reassure the patient, given that 60% of cases resolve without sequelae within 6 months (Table 22.10).
- Prescribe vitamin B12 for a few weeks since this helps the nerve to recover (Tamaddonfard et al. 2014; Horasanli et al. 2017; Hasegawa et al. 2018).
- If the patient does not recover within 2–3 weeks, refer to a neurologist with a full report.
Around 60% of cases resolve within 6 months (Table 22.10). This period is generally accepted, although some authors recommend waiting 2 years (Girard 1979).
In some countries, such as Denmark, these complications are not considered malpractice, but rather accidents or adverse effects of local anesthesia (Hillerup and Jensen 2006). Finally, in cases that do not resolve and cases of pain and severe dysesthesia, the patient should be referred to a pain clinic since surgery does not lead to improvement and may worsen the patient’s condition (Ehrenfeld et al. 1992; Pogrel and Thamby 2000).
Table 22.10 Occurrence of long‐term paresthesia that resolved within 6 months.
| Reference | Sample size | Nerves affected | Resolved |
|---|---|---|---|
| Harn and Durham (1990) | 51 | Lingual | 96% |
| Ehrenfeld et al. (1992) | 9 | Lingual Alveolar inferior |
23% |
| Krafft and Hickel (1994) | 18 | Lingual | 95% |
| Pogrel et al. (1995); Pogrel and Thamby (2000) | 12 | Lingual Alveolar inferior |
33% |
| Hillerup and Jensen (2006) | 22 | Alveolar inferior | 23% |
| Alcaina et al. (2010) | 2 | Alveolar inferior | 100% |
| Garisto et al. (2010) | 108 | Alveolar inferior | 32% |
| Sambrook and Goss (2011) | 8 | Alveolar inferior | 75% |
| 60% |
Alterations of the Sense of Taste
Ten percent of the fibers of the facial nerve (CN VII) leave the nerve 15 mm after exiting the stylomastoid foramen. After a varied course, they join the upper border of the lingual nerve to form the chorda tympani, which also carries special visceral afferent (taste) fibers from the anterior two‐thirds of the tongue as well as general visceral efferent parasympathetic fibers that synapse at the submandibular ganglion and go on to provide innervation of the sublingual and submandibular glands.
Injury to these fibers leads to reduced sense of taste (hypogeusia) on the affected side of the tongue or even total disappearance of the sense of taste (ageusia) for sugar‐sweet, salty, bitter, and acid‐lemon flavors, as assessed using gustometry (Paxton et al. 1994; Hillerup and Jensen 2006). Atrophy of the fungiform papillae on the affected side of the tongue may also be observed (Cowan 1990).
Of note, there have been three cases of involvement of the sense of taste only, with no other nervous abnormality. None of the three recovered within a year (Paxton et al. 1994; Pogrel and Thamby 2000).
Hoarseness
There have been few reports of hoarseness immediately after injection in mandibular block. Hoarseness was accompanied by dysphagia and breathing difficulty, which lasted 2–3 hours before resolving without sequelae. The cause is thought to be recurrent laryngeal nerve block (branch of the vagus nerve, CN X) because of an anatomical variation (Cilasun et al. 2012).
Trismus
Trismus is a limitation of mouth opening. The frequency of trismus caused by local anesthesia is around 1–3% (Table 22.11) and, as we will see, the disorder is associated mainly with mandibular block (Table 22.12), therefore by weighting the average, with respect to all the other dental local anesthesia techniques – mandibular block accounts for 30% of the techniques used (Annex 1) – the frequency of trismus is around 1%.
Local Anesthetic Techniques Implicated in the Development of Trismus
- Mandibular block, which irritates the medial (internal) pterygoid muscle (Stone and Kaban 1979; Stacy et al. 1994). The technique leads to trismus in 3% of cases (Table 22.11) and accounts for 95% of all cases of trismus (Table 22.12).
- In the upper arch, posterior superior alveolar nerve block or the high tuberosity approach. When applied in the area of the maxillary molars, these techniques can irritate the lateral (external) pterygoid muscle (Stone and Kaban 1979; Shaner et al. 2007) and account for 5% of all cases (Table 22.12) as compared to trismus following mandibular block.
Table 22.11 Occurrence of trismus after mandibular block.
| Reference | Sample size (cases/total) | Percentage |
|---|---|---|
| Krafft and Hickel (1994) | 49/12 104 | 0.4% |
| Kaufman et al. (2000) | 3/179 | 1.6% |
| Ridenour et al. (2001) | 1/30 | 3% |
| Mikesell et al. (2005) | 10/114 | 9% |
| Moore et al. (2006) | 2/187 | 1.1% |
| Lenka et al. (2014) | 1/40 | 2.5% |
| Mohajerani et al. (2014) | 2/80 | 2.5% |
| Dubey et al. (2017) | 3/50 | 6% |
| Kiran et al. (2018) | 0/70 | 0% |
| Average | 3% |
Table 22.12 Cases of trismus caused by mandibular block and techniques involving the upper molars
| Reference | Mandibular block | Maxillary techniques involving upper molars |
|---|---|---|
| Campbell (1954) | 1 | 0 |
| Brown (1976a) | 19 | 1 |
| Brooke (1979) | 16 | 0 |
| Stone and Kaban (1979) | 3 | 1 |
| Adam et al. (1995) | 1 | 0 |
| Shaner et al. (2007) | 0 | 1 |
| 40 | 3 | |
| Rounded percentage | 95% | 5% |
Causes of Trismus
- Mechanical injury or irritation of the pterygoid muscles (medial and lateral) by the tip of the needle
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


