
4

Histology, Epithelial Metaplasias, and Noninïammatory and Non-neoplastic Lesions of the Salivary Glands
Like exocrine glands generally, the salivary glands are composed of both acini, which in this case produce saliva, and a duct system, which transports the saliva to the oral cavity. Only serous acini are found in the parotid gland. By contrast, the submandibular and sublingual are mixed glands that are composed of both serous and mucinous acini. With a few exceptions, the minor salivary glands are purely mucinous (Fig. 4–1, p. 46).
 Cellular Composition of Glands
Cellular Composition of Glands
The Parotid
The parotid acini are roughly spherical and are enclosed in loose connective tissue. They are composed of pyramidal cells with their apex at the acinar lumen. The basally situated nucleus is surrounded by a cytoplasm rich in secretory granules, which are more prominent at the apical aspect of the cell. The granules are more or less numerous depending on the secretory activity of the acinar cell. The lumen of the acinus is small and irregular and may not be visible in all acini. Myoepithelial cells are interposed between the serous cells and the basement membrane. They are slender stellate cells with several elongated cytoplasmic processes that surround the acinar cells and are inconspicuous in light microscopic preparations. The myoepithelial cells have a contractile function and propel the saliva into the acinar lumen and into the duct system.
The intercalated ducts are the smallest ducts and are situated within the lobules. Their cuboidal lining cells have nuclei equivalent in size to the acinar cells. They appear larger, however, because the nuclear-cytoplasmic ratio is higher. The intercalated ducts converge to form the intralobular striated ducts. The striated ducts are so named because their tall columnar lining cells have closely spaced, longitudinal basal ridges. The cytoplasm of the striated duct cell stains intensely eosinophilic, owing to the high content of mitochondria (Fig. 4–2, p. 47). Both intercalated ducts and striated ducts are secretory ducts.1 That is, they modify the composition of, as well as transport, the saliva. The large ducts, the interlobular ducts, and the main excretory ducts serve only to transport the saliva.
Interlobular ducts are dispersed in the connective tissue among the lobules. These ducts are lined by a multilayered epithelium composed of tall columnar cells with interposed goblet cells that surmount small cuboidal basal cells. Occasional cells have conspicuous mucinous vacuoles. The main excretory duct, the Stensen’s duct, is lined by similar cells, which at the distal aspect of the duct are replaced by a stratified squamous mucosa that merges with that of the oral cavity at the orifice of the duct.
During embryonic life, lymph nodes develop in close proximity to the parotid gland. They persist as poorly formed nodules or as organized lymph nodes within the adult gland itself. Inclusions of parotid acinar and ductal elements may be found in these intraparotid lymph nodes (Fig. 4–4, p. 47). Lymph nodes are more common in the superficial lobe of the parotid gland and are infrequently found in the deep lobe.2
Fat cells are present among the acinar cells in the parotid gland. Only a few adipocytes are present during childhood and adolescence. They increase during adult life, however, and may be the predominant feature in elderly individuals. Nevertheless, xerostomia is rarely a consequence, as sufficient acini generally remain to meet physiologic demands.
The facial nerve runs through the parotid gland, thereby dividing the gland into superficial and deep lobes. Branches of the facial nerve are routinely noted in microscopic sections. Though rare, melanocytes have been identified in the interlobular duct of the parotid gland.3
The Submandibular Gland
The submandibular gland is composed of tubular acini that are predominantly serous. About 10% of the acini are mucinous. The mucinous cells are large and roughly triangular, with central nuclei and clear cytoplasmic mucin vacuoles of variable size. Groups of serous cells form crescents at the periphery of the mucinous acini (“demilunes”) (Fig. 4–4, p. 47). The acini are arranged in lobules, with intercalated ducts, which are longer than those in the parotid gland, and striated ducts, which are shorter.4 These ducts drain into the main excretory duct, the Wharton’s duct. Although a few lymphoid cells may be present in the connective tissue of the submandibular gland, lymphoid aggregates or lymph nodes are not found normally.5 Fat cells may be present, but they are not as numerous as in the parotid gland. Nerves are not found in the gland parenchyma.
The Sublingual Gland
The subingual gland is also a mixed gland. In contrast to the submandibular gland, however, the secretory lobules of the sublingual gland are primarily mucinous, with a minor component of serous cells. The duct system is similar to that of the other major glands, with the exception that, in addition to the main duct, the Bartholin’s duct, which drains into the submandibular duct, there are several smaller ducts (Rivinus ducts), which open directly onto the floor of the mouth. Fat, lymph nodes, and nerves are not present in the sublingual glands.
Minor Glands
The minor salivary glands are distributed throughout the oral cavity, on the lips, tongue, floor of mouth, buccal mucosa, and soft palate. Notable exceptions are the gingiva and the anterior aspect of the hard palate, which are devoid of these glands. Minor salivary glands are present, however, on the posterolateral aspect of the hard palate and in abundance on the soft palate, including the uvula. The minor glands are located in the submucosa, or among muscle fibers, and are surrounded by a thin fibroconnective tissue. They are not encapsulated. Their alveoli are arranged primarily in lobules. With the exception of the serous Ebner’s (deep posterior salivary glands of the tongue) glands, the minor glands are mucinous or seromucinous. The mucinous cells of the acini are similar to those in the major glands, with basal nuclei and large mucin vacuoles in the cytoplasm. In many minor glands, serous demilunes are present. Occasionally, individual serous cells are found in mucinous acini. Less commonly, serous acini are present. The duct system is far less complex than that of the major salivary glands. The intercalated ducts are longer, and striated ducts are either absent or rudimentary.6 The intercalated ducts are lined by cuboidal cells and drain into intralobular ducts, which are lined by columnar cells. Excretory ducts are lined by columnar cells with interspersed goblet cells that surmount a layer of flattened basal cells. Ciliated cells are found in the excretory ducts of Ebner’s glands. These cells are more common at the most terminal part of the excretory duct. Squamous cells line the ducts at the point of entry into the oral cavity. In older individuals, oncocytes are occasionally encountered amid the columnar cells of the excretory ducts. Melanocytes have been reported in minor glands, in the connective tissue surrounding ducts and acini.7
 Ultrastructure of Cells that Compose Glands
Ultrastructure of Cells that Compose Glands
The most prominent features of serous cells are the membrane-bound secretory granules, which vary in electron density with secretory activity of the cells. The granules are more numerous and more prominent at the apical aspect of the cell, near the lumen (Fig. 4–5, p. 47). The intervening cytoplasm is occupied by organelles that are important for secretory activity, including the endoplasmic reticulum, Golgi vesicles, and mitochondria. The basal plasma membrane is extensively folded. It interdigitates with the nearby cells, thereby increasing its surface area. At the apical aspect of the cell, zonula adherens are present between adjacent acinar cells, whereas desmosomes form the attachments at the basal aspect. Lying between these attachment points are the secretory capillaries, which are continuous with the lumen of the acinus. Numerous microvilli protrude into the secretory capillaries from the adjacent secretory cells. These microvilli are similar to those that protrude into the acinar lumen at the apex of the cells.
In mucinous cells the cytoplasm is filled with mucin vacuoles, and the cytoplasmic organelles (Golgi vesicles, endoplasmic reticulum, and mitochondria) are less numerous than in the serous cells. These organelles occupy the basal zone of the cytoplasm, lateral to the nucleus. Folds in the basal plasma membrane are less prominent than in the serous cells.
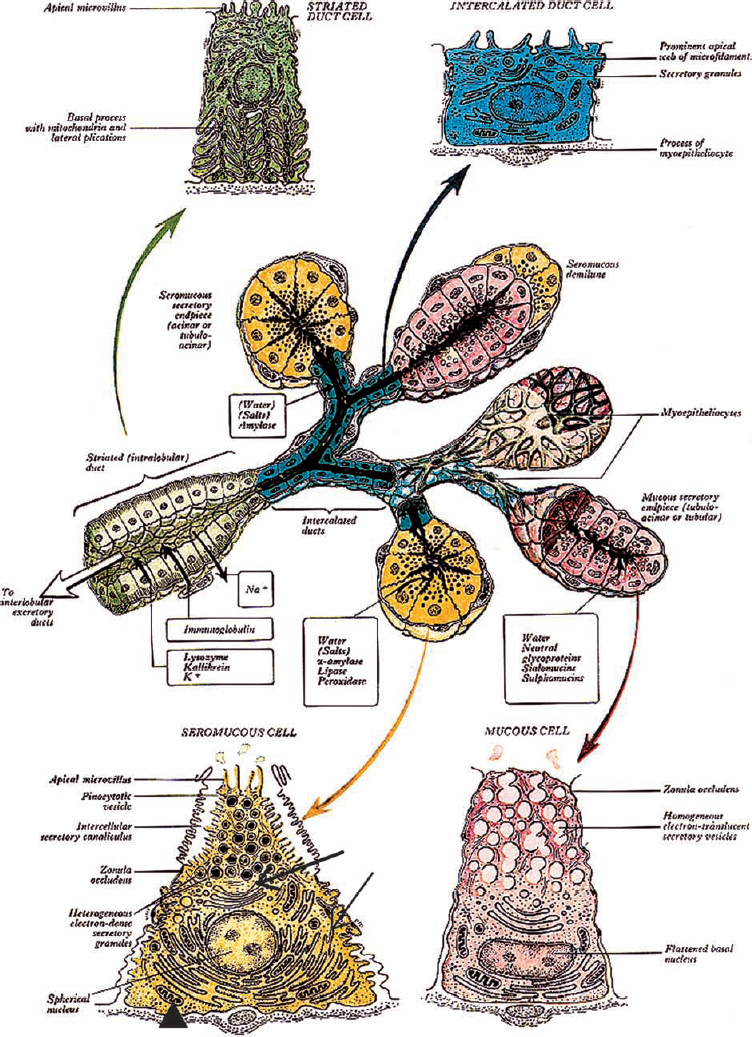
Figure 4-1 Diagram of the architecture of a generalized salivary gland, including ultrastructural details. Solid and outlined black arrows indicate direction of saliva transport. (Seromucous cell, arrowhead: mitochondria, thin arrow: roughendoplasmic reticulum, thick arrow: Golgi complex). (From Williams. Alimentary system. In: Gray H, Williams PL, Bannister LH (eds.). Gray’s Anatomy: The Anatomical Basis of Medicine and Surgery, 38th ed. New York: Churchill Livingstone; 1995, with permission.)
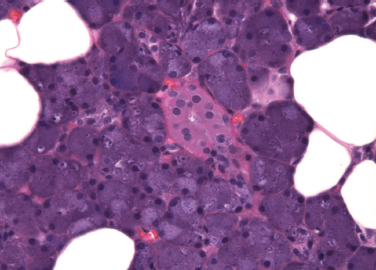
Figure 4-2 Parotid gland. Serous acini surround a striated duct (H&E×400).
Myoepithelial cells are stellate cells, with dendritic processes spread on the surface of the acinar cells. The myoepithelial cells lie between the secretory cells and the connective tissue border of the acinus. These specialized contractile cells have features of both smooth muscle and epithelial cells. They contain myofilaments and actin, as well as pinocytotic vesicles at their basal aspect. Mitochondria, Golgi vesicles, and endoplasmic reticulum are found in the remaining cytoplasm. Myoepithelial cells are connected to the secretory cells by desmosomes.
The few cytoplasmic organelles of intercalated duct cells, principally mitochondria and endoplasmic reticulum, are localized to the basal zone. Occasionally secretory granules may be present, usually in cells close to the acinus. The duct epithelial cells are connected to adjacent cells by junctional complexes and a few desmosomes. Interdigitations are prominent at the lateral aspects of adjacent cells. Myoepithelial cells are present at the periphery of the epithelial cells of the intercalated ducts and occasionally along the striated ducts. They are connected to the epithelial cells by desmosomes (Fig. 4–6).
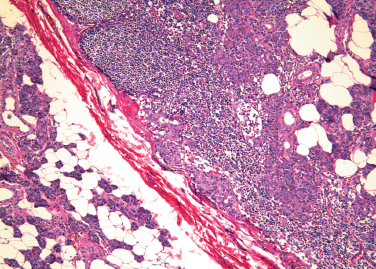
Figure 4-3 Intraparotid lymph node with salivary inclusions (H&E×40).
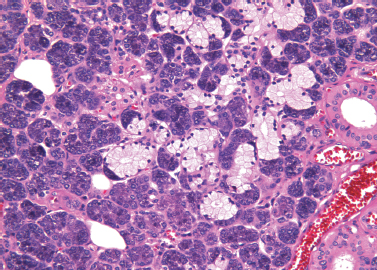
Figure 4-4 Submandibular gland. Serous acinar cells form a crescent at the periphery of a mucinous acinus (H&E×/200).
The columnar cells of the striated ducts are characterized by prominent folds in the basal plasma membrane, which extend apically to the midzone of the cells. The folds also ramify laterally and interdigitate with the adjacent cells, thereby greatly increasing the surface area. They correspond to the striations noted on light microscopy. Between the folds are numerous vertically arranged mitochondria. At the apex, microvilli project into the lumen. Junctional complexes and desmosomes form sites of attachment to adjacent cells.
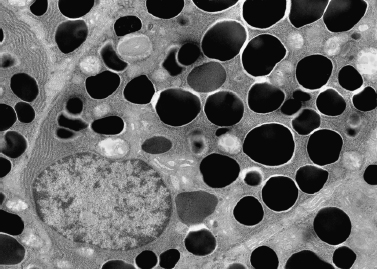
Figure 4-5 Serous cell with numerous secretory granules (electron micrograph × 5000 courtesy of Renato Iozzo, M. D.).
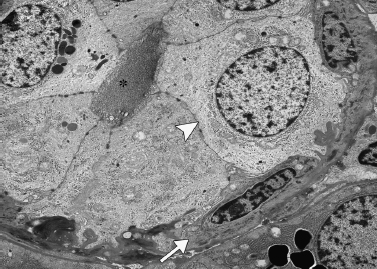
Figure 4-6 Myoepithelial cell (arrow) surrounding epithelial cell (arrowhead) of intercalated duct lumen (asterisk) (electron micrograph/2700 courtesy of Renato Iozzo, M. D.).
The ultrastructural features of the minor glands differ in some respects from those of the major glands.8 One such variation is the absence of basal folds in the serous cells of Ebner’s glands. There are instead numerous folds on the lateral border of the cell.9 Also in Ebner’s glands, many secretory granules are present in the intercalated ducts, as are a well-developed rough endoplasmic reticulum and Golgi apparatus. These features suggest an active secretory role for these cells in the formation of saliva. There are no striated ducts in Ebner’s glands. In all minor glands, intercalated duct cells are rich in mitochondria.
 Immunohistochemistry
Immunohistochemistry
Early reports of the immunohistochemical profile of the salivary glands were frequently conflicting, because of the variability in tissue preparation (frozen vs paraffin sections), the antibodies employed, and the detection methods (immunofluorescence vs peroxidase-antiperoxidase). Based on formalin-fixed, paraffin-embedded tissue, recent reports describe more consistent findings.
Cytokeratins are the intermediate filaments found in epithelial cells. The development of antibodies to specific classes of cytokeratin polypeptides has led to an understanding of the heterogeneity of the salivary epithelium, as expressed in the cytokeratin profile. When exposed to a cocktail of antibodies to high and low molecular weight cytokeratins (AE-1/AE-3), acinar cells stain weakly, but much brighter staining is noted in ductal cells.10 CK-5 and CK-14 are consistently expressed in basal cells in the interlobular and excretory ducts,11 and these cells react strongly to antibodies to high molecular weight cytokeratins (CK-18 and CK-19).12 Myoepithelial cells are positive for antibodies to smooth muscle actin and smooth muscle myosin heavy chain,13,14 as well as CK-14 and CK-17.15 Epithelial membrane antigen (EMA) stains the serous cells and the luminal surface of ductal cells, but it does not stain mucinous cells.16 Although there have been reports to the contrary,17 most investigators have found that S-100 is negative in acinar, ductal, and myoepithelial cells.18,19 Antibodies to vimentin and desmin are consistently nonreactive.
 Hyperplasia, Atrophy, and Regeneration
Hyperplasia, Atrophy, and Regeneration
Adenomatoid Hyperplasia
This is a rare benign disorder of mucinous salivary glands that may mimic a salivary gland neoplasm.20 Patients are asymptomatic, and the nodule is often discovered only incidentally during a dental examination. The hard palate is the most common location, although other intraoral sites are described.21,22 In most cases, biopsy reveals minor salivary glands indistinguishable from normal. A few reports describe glandular crowding or an increased number of glands.23 The cause of this localized hyperplasia is unknown, and excision is curative. No relationship to benign or malignant salivary gland tumors has been shown.
Sialadenosis
Sialadenosis refers to recurrent, painless, usually bilateral enlargement of the major salivary glands, particularly the parotid.24,25 It is associated with endocrine disorders, alcoholism, and metabolic disturbances, including malnutrition and bulimia.26–30 It has been reported as a side effect of certain drugs, particularly antihypertensive agents and antidepressants.31 The etiology of sialadenosis appears to be related to a disorder of the autonomic nervous system. Microscopically, hypertrophy of the acinar cells is striking. The cells are enlarged to 2 to 3 times their normal size, and there is an increase in the intralobular fat. There is no inflammation. Ultrastructurally, myoepithelial cells exhibit features of atrophy, and there are degenerative changes in neural elements.32 Diagnosis may be made by aspiration cytology and morphometry.33 Treatment is directed at the underlying condition. In long-standing cases, atrophy of the gland eventually occurs.
Atrophy
This is a normal consequence of aging salivary glands and is characterized by the loss of acinar cells, which are replaced in turn by fat. Most individuals are asymptomatic because of the considerable normal reserve. Atrophy also occurs in association with pathologic processes, such as compression atrophy adjacent to tumors, and distal to duct obstruction caused by sialoliths or intraductal tumors. With chronic inflammation, atrophy and fibrosis follow acinar destruction. An important iatrogenic cause of atrophy is ionizing radiation.34,35
Regeneration
Regeneration of salivary tissue is limited. Studies of patients following surgery or radiation therapy indicate that both acinar and ductal cells may undergo mitosis, but significant regeneration of acinar tissue does not occur, and acini are replaced by fat and fibrosis. Hyperplasia of the basal cells in larger ducts and squamous metaplasia of ductal cells may be conspicuous. In experimental animals, regeneration of both acinar tissue and ducts can occur following short-term duct obstruction, but little information is available in human subjects in this setting.36,37
 Epithelial Metaplasias
Epithelial Metaplasias
Oncocytic Metaplasia
This is the replacement of normal epithelial cells of both ducts and acini by large, eosinophilic cells (Fig. 4–7). Ultrastructural studies indicate that the cytoplasm of oncocytes is expanded by an increased number of mitochondria, to the virtual exclusion of other organelles. The mitochondria are enlarged and atypical, with a variety of abnormal morphologic features.38
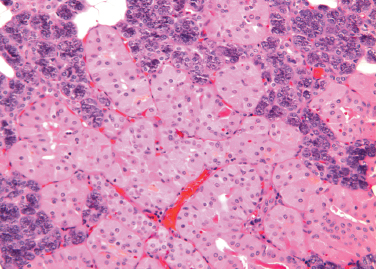
Figure 4-7 Oncocytic metaplasia of parotid acini (H&E × 200).
Oncocytes may occur singly or in clusters. Occasionally, they form tumors, which are usually benign.39,40 Oncocytic metaplasia, as well as tumors of oncocytes, are most common in the parotid. It is less common in the minor salivary glands, but nodular oncocytic hyperplasia may occasionally be found incidentally in biopsy specimens in minor glands throughout the upper aerodigestive tract.
The cause of oncocytic metaplasia is unknown, but it appears to be a degenerative phenomenon related to aging. Rare in individuals younger than 50, oncocytes are frequently seen in older subjects. By the age of 70, they are nearly universal.
Oncocytes are not unique to the salivary glands. They occur in many organs, particularly endocrine organs, including thyroid (Hürthle cells), parathyroid, and adrenal, as well as breast and kidney. Oncocytic tumors may form at these sites as well.
Sebaceous Metaplasia
Sebaceous cells are normally found in the major salivary glands. They are most often seen in the parotid, are infrequently identified in the submandibular glands, and are rare in the sublingual glands.41 Sebaceous cells are also found in the oral mucosa of normal subjects, where they are known as Fordyce’s granules. Rarely, they are present in intraparotid and periparotid lymph nodes.42 In the salivary glands, single cells or groups of sebaceous cells replace the cells of intercalated ducts, or form diverticular outpouchings from the wall of striated ducts or interlobar ducts43,44 (Fig. 4–8). Their morphology is identical to the sebaceous cells found in the dermis: clear cells of variable size, with numerous cytoplasmic vacuoles filled with lipid. Their secretion is extruded into the lumen of the duct and appears in the saliva.
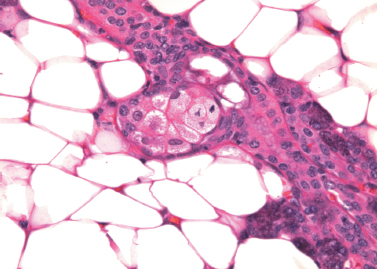
Figure 4-8 Sebaceous cells form a diverticulum from the striated duct in parotid gland (H&E × 400).
The origin and function of sebaceous cells in the salivary glands are unknown. They are present in normal glands and have been described in young children. These observations support the hypothesis that they are a variant of normal differentiation. They have been found in inflamed glands and in those containing tumors, but they appear to be independent of these processes. Heterotopia and metaplasia have both been proposed as possible etiologies. Salivary gland neoplasms with sebaceous differentiation, both benign and malignant, have been described but are rare.10
Squamous Metaplasia
Replacement of acinar and ductal epithelium by squamous cells does not occur in normal glands. Most commonly, squamous metaplasia is a consequence of an inflammatory process or is associated with a calculus.45,46 Necrotizing sialometaplasia is a noteworthy example of exuberant squamous metaplasia. It is an inflammatory response to an ischemic insult affecting minor salivary glands, usually in the oral cavity.47–49Necrotizing sialometaplasia is characterized by ulceration, with necrosis of acini and replacement of the residual acinar and ductal cells by mature squamous cells. The lobular architecture is retained, and spontaneous resolution ensues. Misinterpretation of this reactive response as a neoplastic process has led to serious diagnostic errors.50
Goblet Cell Metaplasia
Goblet cells are normally found in small numbers in the lining epithelium of interlobar ducts. Their numbers increase in inflammatory processes, and in association with calculi and retention cysts. It is speculated that these cells are the origin of mucoepidermoid carcinoma.51
 Accessory and Heterotopic Salivary Gland Tissue
Accessory and Heterotopic Salivary Gland Tissue
An accessory salivary gland is defined as ectopic salivary gland tissue with a duct system. This is in contradistinction to heterotopic salivary gland tissue, in which only acini are present in an abnormal location, but are not associated with a duct system.52 The most common example of accessory salivary tissue is accessory parotid tissue. The usual location for the accessory gland is adjacent to, but distinctly separate from, the main parotid gland, unlike the facial process, which is a small anterior projection of glandular tissue along the duct.53 An accessory parotid gland is usually located anterior to the main parotid gland. It lies superior to the parotid duct, adherent to the masseteric fascia, and in close proximity to the buccal division of the facial nerve. The accessory parotid gland drains into the main parotid duct by one tributary.
Accessory parotid tissue is separated from the main gland by an average of 6 mm and is usually 3 cm or less in size. Histologically, accessory parotid tissue is distinctive, because mucinous cells as well as serous cells may be present in the acini. Autopsy studies have revealed accessory parotid tissue in 21 to 56% of normal adults.54
Any disorder that affects the parotid gland may affect accessory parotid tissue. Interestingly, 1 to 8% of parotid tumors arise from accessory tissue, and a higher percentage of these lesions are malignant (50% as compared with 20–25%).55–57 Symptomatic patients usually present with a painless, mobile midcheek tumor. Confirmation of accessory parotid tissue requires imaging studies to establish that the main gland and mass are indeed separate.
Salivary gland heterotopia refers to the occurrence of salivary tissue at sites other than the normal anatomical locations of the major and minor salivary glands. The most common site of heterotopic salivary tissue is in the cervical lymph nodes.58 Other locations included the middle ear,59 parathyroid60 and thyroid tissue, pituitary glands61 (as a remnant of Rathke’s pouch), the cerebellar pontine angle,62 the soft tissue of the lower neck, and medial to the sternocleidomastoid muscle.63 Rare examples of heterotopic salivary gland tissue in sites remote from the head and neck include the stomach, rectum, and vulva.64–66
Heterotopic salivary gland tissue in cervical lymph nodes is related to the close association of developing lymphoid and salivary tissue in the embryo, a situation that explains the frequent presence of lymph nodes in the parotid and the inclusions of salivary tissue in intraparotid and periparotid lymph nodes. Heterotopic salivary tissue in other head and neck locations is attributed to the persistence and abnormal development of tissue associated with the branchial apparatus and the primitive foregut. Lower cervical heterotopias are thought to result from the failure of closure of the cervical sinus of His associated with the first and second branchial arches.67 The middle ear also develops from the branchial apparatus, and the cranial nerves and their ganglia develop on the dorsal aspect of the branchial arches. The intimate admixture of branches of the facial nerve with ectopic salivary tissue in the middle ear is a consequence of this relationship. The rare occurrence of heterotopic salivary tissue in organs remote from the head and neck remains unexplained.
Neoplasms in heterotopic salivary tissue are rare, but they can pose challenging clinical problems. The presence of salivary gland tumor in cervical lymph nodes may be mistaken for metastatic tumor from a normally situated gland. Alternatively, it may be misclassified as a metastasis from an unknown primary. In either case, inappropriate treatment may result.68–70
 Noninflammatory, Non-neoplastic Disorders
Noninflammatory, Non-neoplastic Disorders
Amyloidosis
Amyloidosis refers to a group of disorders characterized by the localized or systemic deposition in a variety of organs of characteristic extracellular proteins. The fibrillar amyloid proteins are folded as a β-pleated sheet, a feature responsible for their morphologic and staining properties.71 The diverse causes of an amyloid protein deposition include neoplasia, inflammatory processes, and familial syndromes. The clinical presentation and course of the disease depend on the organ(s) affected. Common sites of amyloid deposition include the kidneys, heart, tongue, and brain. Virtually any organ, however, may be affected.72 A diagnosis of amyloidosis depends on the identification of an extracellular amyloid protein in histologic sections, where it appears as an amorphous, eosinophilic substance with a uniform, somewhat glassy hue. The diagnosis is confirmed by staining with Congo red. Amyloid proteins bind this dye, and under polarized light they exhibit a characteristic green birefringence. Ultrastructurally, the protein fibrils are linear, parallel, and 7.5 to 10 nm in diameter.
Historically, amyloidosis was classified as primary, secondary, or localized, depending on the clinical setting in which it arose. It has become evident, however, that this classification is overly simplistic. The current classification system is based on the specific protein responsible for the amyloid deposits.
Involvement of the salivary glands in amyloidosis may be the first clinical evidence of the disorder.73,74 Alternatively, it may be a late feature in a more generalized process.75 Both major and minor salivary glands may be involved. Clinically, xerostomia or bilateral, painless salivary gland enlargement can be present. The frequent (asymptomatic) involvement of the minor glands has been exploited by utilizing biopsy of these glands for diagnosis when amyloidosis is suspected.76
Lipomatosis
Lipomatosis is a condition characterized by an abnormally localized or tumor-like accumulation of intraparenchymal fat tissue. With respect to the salivary glands, a distinction can be readily made between lipomatosis and a lipoma. A lipoma has a fibrous capsule that results in a discrete, localized mass in the salivary tissue. By contrast, lipomatosis is present diffusely throughout the gland. The parotid is the most frequent site; however, lipomatosis of minor salivary glands has been reported.77,78
Lipomatosis can be caused by many localized or systemic conditions. Fatty infiltration of salivary tissue often accompanies aging, associated with atrophy. It has been documented in patients with diabetes mellitus, cirrhosis, chronic alcoholism, malnutrition, and hormonal disturbances.79,80 Recently, parotid lipomatosis has been described in patients positive for the human immunodeficiency virus (HIV) receiving protease inhibitors.81 It has rarely been reported in the pediatric population.82,83
Lipomatosis presents as a soft, diffuse, painless enlargement of the salivary gland, similar to a benign salivary gland tumor. Appropriate diagnostic and imaging studies are indicated, with surgical excision when necessary.
Hemochromatosis
Hereditary hemochromatosis is a common autosomal recessive disorder of iron metabolism characterized by excessive iron absorption and the toxic accumulation of iron in parenchymal cells, particularly the liver, heart, and pancreas. Otolaryngologic manifestations most commonly include xerostomia, due to a loss of salivary gland function as a result of iron deposition.84,85 The definitive diagnostic test is a liver biopsy. However, because of the diffuse systemic nature of this disease, less invasive diagnostic tests have been reported. Labial biopsies have been successful in revealing hemosiderin deposition within the minor salivary glands.86
Cheilitis Glandularis
Cheilitis glandularis is a rare chronic inflammatory condition usually present on the lower lip of adult males.87 Its etiology is unknown. The affected lip is swollen, and on palpation it is nodular, firm, and sometimes tender. Thick saliva can be expressed with digital manipulation. The vermillion border may be everted. Histologic findings are nonspecific and include hyperplasia, hypertrophy, fibrosis, and salivary ductal ectasia. A case associated with squamous cell cancer has been reported.88
REFERENCES
1. Martinez-Madrigal, F Bosq, J Casiraghi, O. Major salivary glands. In: Sternberg, SS, ed. Histology for Pathologists, 2nd ed. Philadelphia: Lippincott-Raven; 1997: 405–429
2. McKean, ME Lee, K McGregor, IA. The distribution of lymph nodes in and around the parotid gland: an anatomical study. Br J Plast Surg 1985; 38: 1–5
3. Takeda, Y. Melanocytes in the human parotid gland. Pathol Int 1997; 47 (8): 581–583
4. Hiatt, JL, Sauk, JJ. Embryology and anatomy of the salivary glands. In: Ellis, GL Auclair, PL Gnepp, DR, eds. Surgical Pathology of the Salivary Glands. Philadelphia: WB Saunders; 1991: 2–9
5. Ciccone, E Truini, M Grossi, CE. Lymphoid complement of the human salivary glands: function and pathology. Eur J Morphol 1998; 36 (Suppl): S252–S256
6. Hand, AR Pathmanathan, D Field, RB. Morphological features of the minor salivary glands. Arch Oral Biol 1999; 44 (Suppl 1): S3–S10
7. Takeda, Y. Existence and distribution of melanocytes and HMB-45- positive cells in the human minor salivary glands. Pathol Int 2000; 50 (1): 15–19
8. Riva, A Loffredo, F Puxeddu, R Testa Riva, F. A scanning and transmission electron microscope study of the human minor salivary glands. Arch Oral Biol 1999; 44: S27–S31
9. Azzali, G Bucci, G Gatti, R Orlandini, G Ferrari, G. Fine structure of the excretory system of the deep posterior (Ebner’s) salivary glands of the human tongue. Acta Anat (Basel) 1989; 136: 257–268
10. Ellis, GL Kauclair, PL. Atlas of Tumor Pathology: Tumors of the Salivary Glands (3rd Series, Fasicle 17). Washingon, DC: Armed Forces Institute of Pathology; 1996
11. Gustafsson, H Kjorell, U Eriksson, A Virtanen, I Thornell, LE. Distribution of intermediate filament proteins in developing and adult salivary glands in man. Anat Embryol (Berl) 1988; 178: 243–251
12. Reis-Filho, JS Simpson, PT Martins, A Preto, A Gartner, F Shcmitt, FC. Distribution of p63, cytokeratins 5/6 and cytokeratin 14 in 51 normal and 400 neoplastic human tissue samples using TARP-4 multitumor tissue microarray. Virchows Arch 2003; 443: 122–132
13. Grandi, D Campanini, N Becchi, G Lazzaretti, M. On the myoepithelium of human salivary glands: an immunochemical study. Eur J Morphol 2000; 38 (4): 249–255
14. Leoncini, P Cintorino, M Vindigni, C, et al. Distribution of cytoskeletal and contractile proteins in normal and tumor bearing salivary and lacrimal glands. Virchows Arch 1988; 412: 329–337
15. Hirano, T Gluckman, JL, deVries, EJ. The expression of avascular smooth-muscle actin in salivary gland tumors. Arch Otolaryngol Head Neck Surg 1990; 116 (6): 692–696
16. Moti, M. Histochemistry of the Salivary Glands. Boca Raton, FL: CRC Press; 2000
17. Prasad, AR Saverna, AT Gown, AM Zarbo, RJ. The myoepithelial immunophenotype in 135 benign and malignant salivary gland tumors other than pleomorphic adenoma. Arch Pathol Lab Med 1999; 123: 801–806
18. Kahn, HJ Baumal, R Marks, A Dardick, I van Nostrand, AWP. Myoepithelial cells in salivary gland tumors: an immunohistochemical study. Arch Pathol Lab Med 1985; 109: 190–195
19. Dardick, I Stratis, M Parks, WR DeNardi, FG Kahn, HJ. S-100 protein antibodies do not label normal salivary gland myoepithelium: histogenic implications for salivary gland tumors. Am J Pathol 1991; 138: 619–628
20. Barrett, AW Speight, PM. Adenomatoid hyperplasia of oral minor salivary glands. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995; 79 (4): 482–487
21. Buchner, A Merrell, PW Carpenter, WM Leider, AS. Adenomatiod hyperplasia of minor salivary glands. Oral Surg Oral Med Oral Pathol 1991; 71 (5): 583–587
22. Chen, YK Lin, CC Lin, LM Yan, YH. Adenomatoid hyperplasia in the mandibular retromolar area: case report. Aust Dent J 1999; 44 (2): 135–136
23. Bryant, C Manisali, BC Barrett, AW. Adenomatoid hyperplasia of palatal minor salivary glands. J Laryngol Otol 1996; 110 (2): 167–169
24. Pape, SA MacLeod, RI McLean, NR Soames, JV. Sialadenosis of the salivary glands. Br J Plast Surg 1995; 48: 419–422
25. Batsakis, JC. Sialadenosis. Ann Otol Rhino Laryngol 1988; 97: 94–95
26. Mandel, L Hamele-Bena, D. Alcoholic parotid sialadenosis. J Am Dent Assoc 1997; 128: 1411–1415
27. Mandel, L Patel, S. Sialadenosis associated with diabetes mellitus: a case report. J Oral Maxillofac Surg 2002; 60: 696–698
28. Mehler, PS Wallace, JA. Sialadenosis in bulimia. Arch Otolaryngol Head Neck Surg 1993; 119: 787–788
29. Coleman, H Altini, M Nayler, S Richards, A. Sialadenosis: a presenting sign in bulimia. Head Neck 1998; 20: 758–762
30. Vavrina, J Muller, W Gebbers, J-O. Enlargement of the salivary glands in bulimia. J Laryngol Otol 1994; 108: 516–518
31. Loria, RC Wedner, H. Facial swelling secondary to inhaled bronchodilaator abuse: catecholamine-induced sialadenosis. Ann Allergy 1989; 62: 289–293
32. Seifert, G. Tumour-like lesions of the salivary glands: the new WHO classification. Pathol Res Pract 1992; 188: 836–846
33. Ascoli, V Albedi, FM DeBlasiis, R Nardi, F. Sialadenosis of the parotid gland: report of four cases diagnosed by fine needle aspiration cytology. Diagn Cytopathol 1993; 9: 151–155
34. Fajardo, LF Berthrong, M. Radiation injury in surgical pathology: 3. Salivary glands, pancreas and skin. Am J Surg Pathol 1981; 5: 279–295
35. Stephens, LC Schultheiss, TE Price, RE Ang, KK Peters, IJ. Radiation apoptosis of serous acinar cells of salivary and lacrimal glands. Cancer 1991; 67: 1539–1543
36. Burgess, KL Dardick, I. Cell population changes during atrophy and regeneration of rat parotid gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998; 85: 699–706
37. Walker, NI Gobe, GC. Cell death and cell proliferation during atrophy of the rat parotid gland induced by duct obstruction. J Pathol 1987; 153: 333–344
38. Johns, ME Regezi, JA Batsakis, JG. Oncocytic neoplasms of salivary glands: an ultrastructural study. Laryngoscope 1977; 87: 862–871
39. Capone, RB Ha, PK Westra, WH, et al. Oncocytic neoplasms of the parotid gland: a 16-year institutional review. Otolaryngol Head Neck Surg 2002; 126: 657–662
40. Blanck, C Eneroth, C-M Jakobsson, PA. Oncocytoma of the parotid gland: neoplasm or nodular hyperplasia? Cancer 1970; 25: 919–925
41. Gnepp, DR. Sebaceous neoplasms of salivary gland origin: a review. In: Sommers, SC Rosen, PP, eds. Pathology Annual (Part 1). East Norwalk, CT: Appleton Century Crofts; 1998: 71–102
42. Linhartova, A. Sebaceous glands in salivary gland tissue. Arch Pathol 1974; 98: 320–324
43. Hartz, PH. Development of sebaceous glands from intralobular ducts of the parotid gland. Arch Pathol 1943; 36: 651–654
44. Meza-Chavez, L. Sebaceous glands in normal and neoplastic parotid glands: possible significance of sebaceous glands in respect to the origin of tumors of the salivary glands. Am J Pathol 1949; 25: 627–645
45. Koudelka, BM. Obstructive disorders. In: Ellis, GL Auclair, PL Gnepp, DR, eds. Surgical Pathology of the Salivary Glands. Philadelphia: WB Saunders; 1991: 26–38
46. Dardick, I Jeans, D Sinnott, NM Wittkuhn, JF Kahn, HJ Baumal, R. Salivary gland components involved in the formation of squamous metaplasia. Am J Pathol 1985; 119: 33–43
47. Abrams, AM Melrose, RJ Howell, FV. Necrotizing sialometaplasia: a disease simulating malignancy. Cancer 1973; 32: 130–135
48. Imbery, TA Edwards, PA. Necrotizing sialometaplasia: literature review and case reports. J Am Dent Assoc 1996; 127: 1087–1092
49. Wenig, BM. Necrotizing sialometaplasia of the larynx: a report of two cases and a review of the literature. Am J Clin Pathol 1995; 103: 609–613
50. Sandmeier, D Bouzourene, H. Necrotizing sialometaplasia: a potential diagnostic pitfall. Histopathology 2002; 40: 200–201
51. Regezi, JA Batsakis, JG. Histogenesis of salivary gland neoplasms. Otolaryngol Clin North Am 1977; 10 (2): 297–307
52. Frommer, J. The human accessory parotid gland: its incidence, nature, and significance. Oral Surg Oral Med Oral Pathol 1977; 43 (5): 671–676
53. Bhide, VN Warshawsky, RJ. Agenesis of the parotid gland: association with ipsilateral accessory parotid tissue. AJR Am J Roentgenol 1998; 170 (6): 1670–1671
54. Toh, H Kodama, J Fukuda, J Rittman, B Mackenzie, I. Incidence and histology of human accessory parotid glands. Anat Rec 1993; 236: 586–590
55. Lewkowicz, A Levy, Y Zeltser, R, et al. Accessory parotid gland masses. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000; 89: 610–612
56. Nemecek, JR Marzek, PA Young, VL. Diagnosis and treatment of accessory parotid gland masses. Ann Plast Surg 1994; 33: 75–79
57. Johnson, FE Spiro, RH. Tumors arising in accessory parotid tissue. Am J Surg 1979; 138: 576–578
58. Warnock, GR Jensen, JL, Kratochvil, FJ. Developmental diseases. In: Ellis, GL Auclair, PL Gnepp, DR, eds. Surgical Pathology of the Salivary Glands. Philadelphia: WB Saunders; 1991: 10–25
59. Kartush, JM Graham, MD. Salivary gland choristoma of the middle ear: a case report and review of the literature. Laryngoscope 1984; 94: 228–230
60. Carney, JA. Salivary heterotopia, cysts, and the parathyroid gland. Am J Surg Pathol 2000; 24 (6): 837–845
61. Schochet, SS Jr McCormick, W Halmi, N. Salivary gland rests in the human pituitary. Arch Pathol 1974; 98: 193–200
62. Curry, B Taylor, C Fischer, A. Salivary gland heterotopia. Arch Pathol Lab Med 1982; 106: 35–38
63. Singer, M Applebaum, E Kyle, L. Heterotopic salivary tissue in the neck. Laryngoscope 1979; 89: 1772–1778
64. Dikman, SH Toker, C. Seromucinous gland ectopia within the prostatic stroma. J Urol 1973; 109: 852–854
65. Weitzner, S. Ectopic salivary gland tissue in the submucosa of the rectum. Dis Colon Rectum 1983; 26: 814–817
66. Marwah, S Berman, ML. Ectopic salivary gland in vulva (choristoma): report of a case and review of literature. Obstet Gynecol 1980; 56: 389–391
67. Youngs, LA Scofield, HH. Heterotopic salivary gland tissue in the lower neck. Arch Pathol 1967; 83: 550–556
68. Ferlito, A Bertino, G Rinaldo, A Mannara, GM Devaney, KO. A review of heterotopia and associated salivary gland neoplasms of the head and neck. J Laryngol Otol 1999; 113: 299–303
69. Snyderman, C Johnson, JT Barnes, EL. Extraparotid Warthin’s tumor. Otolaryngol Head Neck Surg 1986; 94: 169–175
70. Rodgers, GK Felder, H Yunis, EJ. Pleomorphic adenoma of cervical heterotopic salivary gland tissue: case report and review of neoplasms arising in cervical heterotopic salivary gland tissue. Otolaryngol Head Neck Surg 1991; 104: 533–536
71. Glenner, GG. Amyloid deposits and amyloidosis. N Engl J Med 1980; 302: 1283–1291
72. Kisilevsky, R. The amyloidoses. In: Rubin, E Gorstein, F Rubin, R Schwarting, R Strayer, D, eds. Rubin’s Pathology, 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2004: 1186–1200
73. Myssiorek, D Alvi, A Bhuiya, T. Primary salivary gland amyloidosis causing sicca syndrome. Ann Otol Rhinol Laryngol 1992; 101: 487–490
74. Gogel, HK Searles, RP Volpicelli, NA Cornwell, GG. Primary amyloidosis presenting as Sjögren’s syndrome. Arch Intern Med 1983; 143: 2325–2326
75. Delevaux, I Andre, M Amoura, Z Kemeny, J-L Piette, J-C Aumaitre, O. Concomitant diagnosis of primary Sjögren’s syndrome and systemic AL amyloidosis. Ann Rheum Dis 2001; 60: 694–695
76. Hachulla, E Janin, A Flipo, RM, et al. Labial salivary gland biopsy is a reliable test for the diagnosis of primary and secondary amyloidosis. Arthritis Rheum 1993; 36: 691–697
77. Walts, A Perzik, S. Lipomatous lesions of the parotid area. Arch Otolaryngol 1976; 102: 230–232
78. Saleh, H Harmse, J Michie, B, et al. Lipomatosis of the minor salivary glands. J Laryngol Otol 1998; 112: 895–897
79. Davidson, D Leibel, BS Berris, B. Asymptomatic parotid gland enlargement in diabetes mellitus. Ann Intern Med 1969; 70: 31–38
80. Scott, J Burns, J Flower, E. Histopathological analysis of parotid and submandibular glands in chronic alcohol abuse: a necropsy study. J Clin Pathol 1988; 41: 837–840
81. Olive, A Salavert, A Manriquez, M Clotet, B Moragas, A. Parotid lipomatosis in HIV positive patient: a new clinical disorder associated with protease inhibitors. Ann Rheum Dis 1998; 57 (12): 749
82. Esposito, C Califano, L D’Armiento, M Longo, F. Lipomatosis of the parotid gland of a child. Br J Plast Surg 2000; 53 (8): 699–701
83. Holland, AJ Baron-Hay, GS Brennan, BA. Parotid lipomatosis. J Pediatr Surg 1996; 31 (10): 1422–1423
84. Ward-Booth, P Ferguson, MM MacDonald, DG. Salivary gland involvement in hemachromatosis. Oral Surg 1981; 487–488
85. Takeda, Y Ohya, T. Sicca symptom in a patient with hemochromatosis: minor salivary gland biopsy for differential diagnosis. Int J Oral Maxillofac Surg 1987; 16 (6): 745–748
86. Verma, S. Chelitis glandularis: a rare entity. Br J Dermatol 2003; 148: 362
87. Rogers, RS 3rd Bekic, M. Diseases of the lips. Semin Cutan Med Surg 1997; 16 (4): 328–336
88. Cohen, DM Green, JG Diekmann, SL. Concurrent anamolies: cheilitis glandularis and double lip. Report of a case. Oral Surg Oral Med Pathol 1988; 66: 397–399
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


