
6

Histology and Pathology of Sialolithiasis
The background to our present understanding of sialolithiasis starts in 1896, when Küttner1 published his observations on two patients who had suffered from chronically swollen submandibular glands that had attracted a clinical diagnosis of malignancy. His microscopic examination revealed these were not neoplastic, but chronically inflamed. He found a sialolith in one of the cases, and was of the opinion that the chronic sialadenitis was primary and arose by inflammation that ascended Wharton’s duct from the mouth. He considered that the sialolith was secondary and arose from an irregularity in the lining of a duct, in a lobule whose excretory duct was compressed by inflammatory swelling, or in a bacterial deposit. Küttner’s seminal publication established chronic submandibular sialadenitis as an entity, which became known as Küttner’s tumor in continental Europe. It also made a contribution to the natural history of chronic sialadenitis and sialolithiasis that has recently been shown to be essentially correct.
Thus chronic sialadenitis and sialolithiasis were interrelated from the beginning, and they need to be considered together to understand the etiology and pathogenesis of either. They were reviewed in 1970 in the final edition of Thoma’s Oral Pathology.2 On the etiology of chronic sialadenitis, there was little more than salivary calculi are almost exclusively the cause of chronic submandibular sialadenitis, whereas hyposialia of the parotid gland is the most important prerequisite for chronic recurrent parotitis. The notion that sialolithiasis is secondary to sialadenitis had been dropped, and the etiology of sialolithiasis was divided into numerous theoretical causes that included mechanical, chemical, inflammatory, and neurohumoral. These are complex and need not be described here because extensive research has now led to a clear understanding of the natural history of chronic sialadenitis and sialolithiasis.
 Natural History of Chronic Sialadenitis and Sialolithiasis
Natural History of Chronic Sialadenitis and Sialolithiasis
Studies of Experimental Sialomicrolithiasis in Animals
Much of the research into the natural history of chronic sialadenitis and sialolithiasis has been experimental. In the early 1960s, Seifert started to use rat as an experimental model to produce microscopic concretions, called sialomicroliths, and sialadenitis in the submandibular and parotid glands. He and his colleagues3 continued these investigations over the next 2 decades. These investigations included combinations of hypercalcemia and very high doses of stimulatory agents such as isoprenaline (isoproterenol). The unraveling of the underlying biological processes was an important step in reaching an understanding of the etiology and pathogenesis of chronic sialadenitis and sialolithiasis.4 Isoprenaline given in repeated high doses soon produces a great increase in the size and weight of the submandibular and parotid glands in the rat as a result of hyperplasia and hypertrophy of the acinar cells. The acinar enlargement is sufficient to result in compression of the intraglandular ducts. Every dose of isoprenaline is followed by an explosive release of secretory material from the acinar cells, which is unable to flow freely through the lumina of the ducts that are partially obstructed by compression, and the resultant increase of pressure in the lumina of the acini damages acinar cells. This partial obstruction thus results in the stagnation of secretory material that is rich in calcium because of the hypercalcemia. Cell membranes contain phospholipids, which, when exposed because of damage to the membranes, are potent nucleators of calcification.5–8 Calcium precipitates on these exposed phospholipids to form sialomicroliths that cause ductal obstruction and sialadentitis.4
The concept that sialomicroliths could lead to sialadenitis was enhanced by the discovery of sialomicroliths in chronic submandibular sialadenitis9 and in normal submandibular glands10 in humans. Because of the possible importance of sialomicroliths in the etiology of sialadenitis and sialolithiasis, an extensive investigation of an archive of normal and experimentally affected salivary glands of cat, in which sialomicroliths had been observed, was undertaken.11–19 The archival material consisted of ideally preserved specimens of parotid, submandibular, and sublingual glands of cat that comprised normal control glands and glands that had been variously subjected to ductal ligation, stimulation of the parasympathetic and sympathetic nerves, para-sympathectomy, and sympathectomy.
Sialomicroliths were detected in 1% of the normal parotids, 10% of the normal submandibular glands, and 27% of the normal sublingual glands. There was a greatly increased occurrence of sialomicroliths in the parasympathectomized submandibular glands, in which they were found in 76%. There were no significant changes in the occurrence of sialomicroliths in any of the other experimental glands. This led to the important realization that the reduction of secretory stimulation resulting from the parasympathectomy had caused the formation of a pathological quantity of sialomicroliths, which led to the suggestion that secretory inactivity was the cause of sialomicrolithiasis in humans.
Sialomicroliths were found in autophagosomes in the acinar secretory cells of the submandibular and sublingual glands of cat, where they arose from degradation of redundant secretory granules and other organelles by the process of autophagy. These secretory granules contain a large amount of sequestered calcium that is present as a cationic shield to allow the condensation of the acidic glycoprotein present in the secretory material. This calcium is released in an ionized form during the normal release of secretory material from secretory granules or during the degradation of secretory granules. The phospholipid of cell membranes that are degraded during autophagy of organelles becomes exposed and is a potent nucleator of calcification.5–8 The ionized calcium precipitates on the exposed phospholipid to form calcified sialomicroliths in the autophagosomes, and thereby the cell is saved from poisoning by an overwhelming release of ionized calcium. Sialomicrolithiasis thus appears to be a physiological process to safeguard the cell (S. Y. Ali, personal communication, 1991). Sialomicroliths were also found in lumina, where they could arise by expulsion from acinar cells or by formation in stagnant secretory material (Figs. 6–1 and 6–2). There appears to be a turnover of sialomicroliths, and means of removal include discharge from acinar and ductal cells luminally to be flushed away in the saliva, and laterally into intercellular spaces and basally into the stroma to be engulfed by macrophages. The lysosomal enzymes and acidity of the phagosomes would be overwhelmed during the formation of sialomicroliths, and the restoration of enzymes and acidity by fusion with primary lysosomes would enable a controlled degradation of sialomicroliths and a harmless gradual release of calcium to occur.
A pathological increase of sialomicroliths occurs only if the balance between formation and removal is disturbed. This happens in the parasympathectomized submandibular glands, in which secretory inactivity leads to autophagy of secretory granules and stagnation of secretory material in lumina, thereby facilitating the formation of sialomicroliths when the sequestered calcium is released as ionized calcium in the presence of phospholipid. However, complete obstruction of the feline submandibular gland by ductal ligation is followed by autophagy in acinar cells and stagnation of secretory material together with degeneration of cell membranes, but there is no increase of sialomicroliths in these glands. The answer to this appears to involve the macrophages, which are present in increased numbers in ligated glands, and may scavenge sialomicroliths so effectively that there is no increase. Also, there may be insufficient calcium in the ligated glands for an increased formation of calcified sialomicroliths, for a decrease of calcium has been detected in ligated salivary glands.
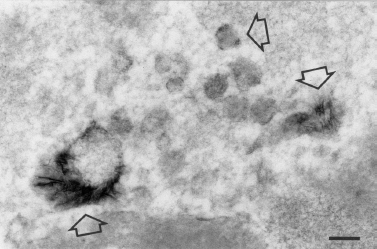
FIGURE 6-1 Needle-shaped crystals of hydroxyapatite (arrows) are associated with membranous debris in the lumen of a duct. Submandibular gland of cat 14 days after para-sympathectomy. Tissue immersion fixed in glutaraldehyde and formaldehyde and subsequently in osmium tetroxide, and section stained with lead citrate. ×69,600; bar=0.1 μm. (Electronmicrograph courtesy of A. Triantafyllou, M. D.)
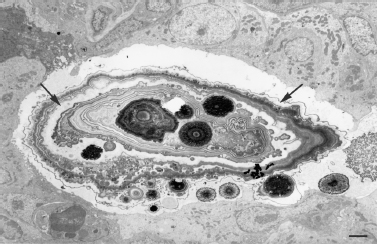
FIGURE 6-2 A large sialomicrolith is present in the lumen of a duct and consists of lamellae and cores variously arranged around single cores, groups of cores and groups of lamellae, and secretory material (arrows), which indicates that many small sialomicroliths had accreted to form the large sialomicrolith. The cores and lamellae consist either of crystals, which are very dense, or granular material, which is less dense. Small sialomicroliths are present in surrounding secretory material. Submandibular gland of cat 21 days after parasympathectomy and sympathetically stimulated. Tissue immersion fixed in glutaraldehyde and formaldehyde and subsequently in osmium tetroxide, and section stained with lead citrate, ×2100; bar = 2 μm. (Electronmicrograph courtesy of A. Triantafyllou, M. D.)
Partial but not complete obstruction is associated with an increase of sialomicroliths, which is seen in the salivary glands of rat stimulated by very high doses of isoprenaline in which partial obstruction is an essential factor.4 Stimulation of a partially obstructed gland would lead to a release of secretory material from acinar cells that overwhelms the ability of the partially obstructed duct to allow it egress. The resultant acute blockage and increase of pressure in the lumina would lead to a large amount of stagnant secretory material rich in calcium that can precipitate on phospholipid exposed in membranes damaged by the increase of pressure.
Although sialomicroliths were produced experimentally, there was never any formation of sialoliths. However, the feline submandibular glands were only examined up to 42 days after parasympathectomy, and whether longer periods would have led to sufficient accretion of sialomicroliths to produce sialoliths or whether there would have been insufficient dilatation of ducts to allow this remains unknown. However, the feline model produced a wealth of information on salivary calcification that is applicable to the human condition.
Studies of Sialomicrolithiasis in Humans
Scott10,20 performed an extensive morphometric investigation of the effects of aging on the submandibular gland in humans in the 1970s and found that the parenchyma of normal glands contained sialomicroliths and atrophic foci. Sialomicroliths were associated with the atrophic foci (Figs. 6–3), and the occurrence of both increased with age. Scott’s finding that sialomicroliths occurred in normal submandibular glands was a breakthrough in the search for the link between normal glands and sialadenitis and sialolithiasis. It was pursued by an investigation21 in which sialomicroliths were found in all normal submandibular glands and 20% of normal parotids. They were found in parenchymal cells, lumina, and stroma, and appeared to be formed similarly to those in the feline glands. The finding of sialomicroliths in all the submandibular glands and in only a minority of the parotids corresponds to a higher concentration of calcium in the submandibular gland.22
The first reported observation of sialomicroliths in humans was in 1965 by Tandler,23 who observed them with the electron microscope in ductal lumina of a submandibular gland and considered them to be inchoate sialoliths. This suggestion led to a search for sialomicroliths in chronic submandibular sialadenitis, and they were found in all cases.24 One case was examined with the electron microscope together with a case of parotid sialadenosis.25 Sialomicroliths in the case of sialadenitis were well calcified and were found in the stroma, parenchyma, and lumina. They were found less often in the case of sialadenosis, in which they were poorly calcified, and were found in the stroma associated with necrotic acinar cells and in the parenchyma. Sialomicroliths were found in macrophages in both sialadenitis and sialadenosis. The finding of sialomicroliths in the case of parotid sialadenosis contrasts with the rarity of sialomicroliths in normal parotids and appears to relate to the decreased secretory activity of sialadenosis. This would lead to autophagy of secretory granules and release of sequestered calcium, which precipitates on exposed phospholipid to form sialomicroliths in phagosomes. However, when this process is overwhelmed, the released calcium reaches a concentration that kills the cell and then precipitates onto phospholipid exposed in the degenerating membranes of the dead cell, which accounts for the sialomicroliths in necrotic cells. There is also a turnover of sialomicroliths with removal by scavenging macrophages as in cat. The differences in the degree of calcification of the sialomicroliths relate to the amount of calcium sequestered in the glycoprotein of the secretory granules, which is lower in the parotid.22
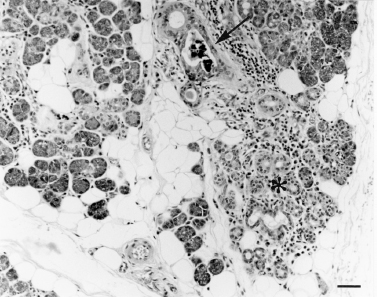
FIGURE 6-3 A group of sialomicroliths (arrow) obstructs the lumen of a striated duct that leads from an inflamed atrophic focus (asterisk). Normal submandibular gland removed during surgery. Section stained with hematoxylin and eosin, × 110; bar = 50 μm.
These morphological investigations did not reveal a development of sialoliths from sialomicroliths, and the relation between sialoliths and sialomicroliths was finally established through the investigation of a large series of cases of chronic submandibular sialadenitis by a combination of morphological, clinical, and epidemiological techniques.
Studies of Sialolithiasis in Humans
Seifert and Donath9 published an important clinicopathological investigation of 349 cases of Küttner’s tumor in 1977. They widened its scope from the classic features of chronic sclerosing submandibular sialadenitis described by Küttner1 in 1896 to encompass cases with only minor histological changes, and thereby made a valuable contribution to our understanding of the etiology and pathogenesis of the condition. They considered that Küttner’s tumor or chronic submandibular sialadenitis starts as a secretory disturbance in which there is a condensation of secretory material in lumina with the formation of both calcified and purely organic sialomicroliths that lead to obstruction followed by inflammation, increasing destruction of the parenchyma, fibrosis of the lobules, and a conspicuous lymphocytic infiltrate with lymphoid follicles. The decreased secretory activity as a result of the atrophy favors ascending ductal invasion by microbes that sustain the inflammation; thus a vicious circle ensues, and the manifest sialolith is the final stage.
This concept of chronological progress through increasingly severe changes was challenged in 1981 by Isacsson et al,26 who found no relation between the severity of the changes in the glands and the duration of symptoms, and considered sialoliths to be the main etiological factor of chronic submandibular sialadenitis and not secondary to it. However, in a subsequent thorough clinicopathological investigation27 of chronic submandibular sialadenitis in which 154 cases were evaluated and statistically analyzed for 18 different clinical and histological features, which encompassed the wide range of histological appearances established by Seifert and Donath9 as part of chronic submandibular sialadenitis, it was found that sialoliths, atrophy, fibrosis, parenchymal inflammation, lymphoid germinal centers, mucous and ciliary metaplasia, extravasation of saliva, and accumulation of glycosaminoglycan are related to the total infiltrate of inflammatory cells, which appears to be of great importance in the etiology and pathogenesis of chronic submandibular sialadenitis. The total infiltrate of inflammatory cells, atrophy, fibrosis, and sialoliths, which had been present at some time in the gland or duct of 68% of the cases, was found to be related to the duration of symptoms, which supports Seifert and Donath’s concept of chronological progress through increasingly severe histological changes with the secondary formation of sialoliths. Sialomicroliths, in contrast, were found to be related to age as in normal glands and not to duration of symptoms or to sialoliths, which was a surprise, as the suggestion had been made that sialomicroliths occasionally impacted and accreted to form sialoliths.11,21,24 However, the investigations on the feline glands12,17 revealed the importance of secretory inactivity in the production of sialomicroliths, and together with the results of clinical and pathological investigations,27–29 led to the following understanding of the natural history of sialolithiasis (Tables 6–1).
Secretory inactivity in a normal gland leads simultaneously to increased formation of sialomicroliths and ascent of the main duct by commensal microbes. Impaction of a sialomicrolith in a small intraglandular duct causes focal obstructive atrophy (Figs. 6–3). Microbes proliferate in atrophic parenchyma where they are protected from the flushing and microbicidal activity of saliva and from systemic immunity by the associated fibrosis. The diffusion of their waste products and local invasion cause inflammation, the fluid exudate of which compresses surrounding parenchyma and causes further atrophy, which is associated with further invasion by microbes. The process spreads to involve more of the gland until inflammatory swelling and fibrosis secondary to inflammation compress interlobular ducts, which causes partial obstruction that leads to dilatation and intraluminal stagnation of secretory material rich in calcium that precipitates onto the phospholipid exposed in degenerate membranes (Fig. 6–4); accretion occurs until a sialolith is formed (Figs. 6–5). This causes further obstructive atrophy and reduction in salivary flow, all of which facilitate further ascent by microbes. The process progresses, and the gland becomes increasingly inflamed, atrophied, and fibrosed.
Table 6-1 Natural History of Chronic Sialadenitis and Sialolithiasis
| Secretory inactivity in normal gland |
| Accumulation of sialomicroliths causes foci of obstructive atrophy |
| Microbes ascend main duct and proliferate in foci of obstructive atrophy |
| Inflammation with fluid exudate |
| Compression of surrounding parenchyma with further atrophy |
| Further ascent by and proliferation of microbes |
| Further inflammation with fluid exudate |
| Compression of large duct with partial obstruction |
| Stagnation of secretory material and released calcium |
| Formation of sialolith as calcium precipitates on phospholipid of degenerate membranes |
| Further obstructive atrophy, ascent by microbes, and inflammation |
There has not been a comparable investigation into the etiology and pathogenesis of parotid sialolithiasis, and the etiology of chronic parotitis is unclear.30 Sialomicroliths and atrophic foci occur in the normal parotid,21,31 and it is likely that the etiology and pathogenesis of parotid sialolithiasis are similar to those of submandibular sialolithiasis. An additional obstructive factor is an albuminous coagulum that forms when plasma proteins leak into the lumina of inflamed glands.30
Sialolithiasis of the minor salivary glands is relatively rare, and the peak incidence is later than that of the submandibular gland and is in the fifth to eighth decades.32–34 The reason for this relates to the spontaneous secretion of the minor salivary glands that occurs in the absence of nervous stimulation, in contrast to the submandibular and parotid glands in which secretion stops in the absence of nervous stimulation.35 Degenerative changes occur in the minor glands with age and involve acinar atrophy, the occurrence of sialomicroliths in ductal lumina, and an increase of inflammatory cells.36 This will eventually lead to stagnation of secretory material that is rich in calcium22 and the formation of sialoliths.
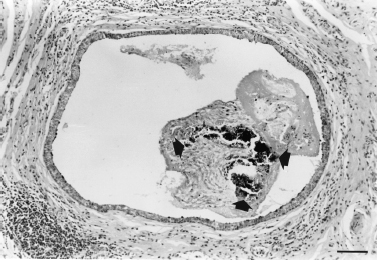
FIGURE 6-4 An inchoate lith consists of foci of calcification (between the three arrows) mixed with secretory material, inflammatory cells, and cellular debris in the dilated lumen of an interlobular collecting duct. Chronic submandibular sialadenitis with sialolithiasis. Section stained with hematoxylin and eosin, × 70; bar = 100 μm.
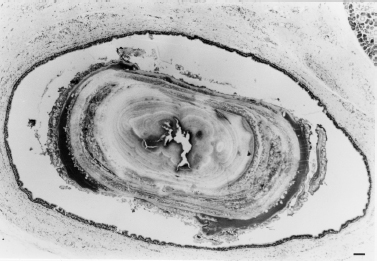
FIGURE 6-5 A sialolith obstructs the dilated lumen of an interlobular collecting duct and contains many lamellae and several central cores, which indicates that accretion had occurred. Chronic submandibular sialadenitis with sialolithiasis. Tissue decalcified and section stained with hematoxylin and eosin, × 27; bar = 100 μm
 The Structure and Composition of Sialomicroliths
The Structure and Composition of Sialomicroliths
A sialomicrolith is defined as a concretion in a salivary gland that can only be seen microscopically and is most often calcified.14 Sialomicroliths range from consisting mainly of crystals of calcium and phosphorus in the form of apatite to consisting mainly of granular material, which is condensed secretory material, without crystals.11,14,21,24,25 Some of the sialomicroliths in the parasympathectomized feline submandibular glands are of a florid appearance (Figs. 6–2) and consist of complex mixtures of crystals and granular material, which indicates that they grew and fused by accretion and that the presence or absence of crystals relates to the local concentration of calcium at the time that a particular part is forming. Similarly, the sialomicroliths in chronic submandibular sialadenitis are well calcified, which corresponds to a higher concentration of calcium in the submandibular gland, whereas those found in parotid sialadenosis are poorly or not calcified, which corresponds to a lower concentration of calcium in the parotid.22
 The Structure of Sialoliths
The Structure of Sialoliths
A sialolith (Fig. 6–5) is defined as a concretion in a salivary gland or main duct that can be seen with thenaked eye and is most often calcified. Sialoliths share many features with sialomicroliths, including great variation in structure and in the content of mineral and organic matrix, the latter of which has been found to vary in different parts of sialoliths from 23 to 100% by volume of the part analyzed,37 as well as being the sole component of some sialoliths.38 The mineral component has been found to be proportional to the size of sialoliths, which indicates that there is increasing mineralization of the organic matrix with time.39 The organic matrix contains glycoprotein40 and lipids derived from secretory material and cell membranes,5–8,41 which indicates a process of formation similar to that of sialomicroliths, namely a condensation of stagnant secretory material and a precipitation of the sequestered calcium released in an ionized form on to phospholipid exposed in degenerate cell membranes to form apatite (Fig. 6–4). This is facilitated by reduced amounts of phytate and magnesium, which are potent inhibitors of apatite crystallization and have been found to be present in reduced amount in the saliva of patients with mineralized sialoliths.38
The great variation in the structure of sialoliths (Fig. 6–5) is manifest as cores, which are not always present, are single or multiple, and vary from purely organic to heavily calcified; lamellae, which range from being present throughout all but the core to being absent; spheroidal bodies that appear to be condensed secretory material or lipid; bacteria, which are not usually seen in the cores and may be calcified; and a surface coating of condensed secretory material and cellular debris.37,38,41–49 These variations are caused by variations in the microenvironment between glands and within glands at different times, and parts of sialoliths may change as the microenvironment changes. The core is formed initially, yet it sometimes exhibits a substructure, which indicates a formation by accretion and fusion of smaller sialoliths. This process possibly also includes sialomicroliths.
Direct involvement of bacteria in the formation of sialoliths has been suggested,45,47 although the absence of bacteria in sialomicroliths indicates that bacteria are not necessary for calcification and the formation of sialoliths. It appears that bacteria are involved later when the partial obstruction caused by a sialolith enables them to ascend more easily from the mouth to reach and colonize the surface of the sialolith.
Sialoliths of a crystalloid structure have been described in the parotid48 and minor salivary glands.49 They appear to consist of precipitated protein and to be formed by condensation of stagnant secretory material.
 The Composition of the Mineral of Sialoliths
The Composition of the Mineral of Sialoliths
The microenvironment determines the type of salt formed and the degree of calcification. The saliva of patients with calcified sialoliths contains more calcium than that of controls and of patients with purely organic sialoliths.38
Submandibular sialoliths5,37,38,42,43,50–52 usually contain apatite, Ca5(PO4)3OH, as the principal mineral and often sole mineral. Whitlockite, Ca3(PO4)2, is the next most common mineral, appears to be found only in sialoliths from Wharton’s duct, is often mixed with apatite, is often localized centrally and in well-mineralized areas, and is not present in lamellae. Octacalcium phosphate, Ca8H2(PO4)6.5H2O, and brushite, CaHPO4.2H2O, are seldom present.
Parotid sialoliths6,50,51 commonly consist of octacalcium phosphate mixed with apatite and sometimes also whitlockite, and consist solely of apatite less often than submandibular sialoliths.
 Conclusion
Conclusion
The synthesis of many experimental and clinical investigations has led to a radical change in our understanding of the natural history of chronic sialadenitis and sialolithiasis, the etiology and pathogenesis of which are interrelated and inseparable, and has supported Ku¨ ttner’s1 notion that sialolithiasis is secondary to sialadenitis. The new understanding indicates the importance of good salivary secretory activity not only as a therapy but also as a prophylactic against chronic sialadenitis and sialolithiasis. Another important factor is phytate,38 which inhibits the formation of sialoliths by chelating the released ionized calcium; a diet with a reasonable content of phytate, which is found in seeds, would appear to be prophylactic.
REFERENCES
1. Küttner, H. [On the inflammatory tumors of the submandibular salivary gland.] Beitr Klin Chir 1896; 15: 815–828
2. Rauch, S Gorlin, RJ. Functional disorders; diagnostic aids; developmental anomalies; inflammatory disorders; sialadenosis; sialolithiasis. In Gorlin, RJ Goldman, HM, eds. Thoma’s Oral Pathology, 6th ed. St Louis: Mosby; 1970: 962–1070
3. Westhofen, M Schäfer, H Seifert, G. Calcium redistribution, calcification and stone formation in the parotid gland during experimental stimulation and hypercalcaemia: cytochemical and X-ray microanalytical investigations. Virchows Arch A Pathol Anat Histopathol 1984; 402: 425–438
4. Harrison, JD Epivatianos, A. Production of microliths and sialadenitis in rats by a short combined course of isoprenaline and calcium gluconate. Oral Surg Oral Med Oral Pathol 1992; 73: 585–590
5. Boskey, AL Boyan-Salyers, BD Burstein, LS Mandel, ID. Lipids associated with mineralization of human submandibular gland sialoliths. Arch Oral Biol 1981; 26: 779–785
6. Boskey, AL Burstein, LS Mandel, ID. Phospholipids associated with human parotid gland sialoliths. Arch Oral Biol 1983; 28: 655–657
7. Slomiany, BL Murty, VLN Aono, M Slomiany, A Mandel, ID. Lipid composition of the matrix of human submandibular salivary gland stones. Arch Oral Biol 1982; 27: 673–677
8. Slomiany, BL Murty, VLN Aono, M Slomiany, A Mandel, ID. Lipid composition of human parotid salivary gland stones. J Dent Res 1983; 62: 866–869
9. Seifert, G Donath, K. [On the pathogenesis of Kü ttner’s tumor of the submandibular gland. Analysis of 349 cases of chronic Sialadenitis of the submandibular gland.] HNO 1977; 25: 81–92
10. Scott, J. The prevalence of consolidated salivary deposits in the small ducts of human submandibular glands. J Oral Pathol 1978; 7: 28–37
11. Epivatianos, A Harrison, JD Garrett, JR Davies, KJ Senkus, R. Ultrastructural and histochemical observations on intracellular and luminal microcalculi in the feline sublingual salivary gland. J Oral Pathol 1986; 15: 513–517
12. Triantafyllou, A Harrison, JD Garrett, JR Kidd, A. Increase of microliths in inactive salivary glands of cat. Arch Oral Biol 1992; 37: 663–666
13. Triantafyllou, A Harrison, JD Garrett, JR. Microliths in normal salivary glands of cat investigated by light and electron microscopy. Cell Tissue Res 1993; 272: 321–327
14. Triantafyllou, A Harrison, JD Garrett, JR. Analytical ultrastructural investigation of microliths in salivary glands of cat. Histochem J 1993; 25: 183–190
15. Harrison, JD Triantafyllou, A Garrett, JR. The effect of sympathectomy on the occurrence of microliths in salivary glands of cat as studied by light and electron microscopy. Arch Oral Biol 1993; 38: 79–84
16. Harrison, JD Triantafyllou, A Garrett, JR. The effects of obstruction and secretory stimulation on microlithiasis in salivary glands of cat: light and electron microscopy. Virchows Arch B Cell Pathol Incl Mol Pathol 1993; 64: 29–35
17. Triantafyllou, A Harrison, JD Garrett, JR. Production of salivary microlithiasis in cats by parasympathectomy: light and electron microscopy. Int J Exp Pathol 1993; 74: 103–112
18. Harrison, JD Triantafyllou, A Baldwin, D Garrett, JR Schäfer, H. Histochemical and biochemical determination of calcium in salivary glands of cat. Histochemistry 1993; 100: 155–159
19. Harrison, JD Triantafyllou, A Garrett, JR. Ultrastructural localization of microliths in salivary glands of cat. J Oral Pathol Med 1993; 22: 358–362
20. Scott, J. The incidence of focal chronic inflammatory changes in human submandibular salivary glands. J Oral Pathol 1976; 5: 334–346
21. Epivatianos, A Harrison, JD. The presence of microcalculi in normal human submandibular and parotid salivary glands. Arch Oral Biol 1989; 34: 261–265
22. Harrison, JD Triantafyllou, A Baldwin, D Schäfer, H. Histochemical and biochemical determination of calcium in salivary glands with particular reference to chronic submandibular sialadenitis. Virchows Arch A Pathol Anat Histopathol 1993; 423: 29–32
23. Tandler, B. Electron microscopical observations on early sialoliths in a human submaxillary gland. Arch Oral Biol 1965; 10: 509–522
24. Epivatianos, A Harrison, JD Dimitriou, T. Ultrastructural and histochemical observations on microcalculi in chronic submandibular sialadenitis. J Oral Pathol 1987; 16: 514–517
25. Triantafyllou, A Harrison, JD Donath, K. Microlithiasis in parotid sialadenosis and chronic submandibular sialadenitis is related to the microenvironment: an ultrastructural and microanalytical investigation. Histopathology 1998; 32: 530–535
26. Isacsson, G Ahlner, B Lundquist, PG. Chronic sialadenitis of the submandibular gland: a retrospective study of 108 cases. Arch Otorhinolaryngol 1981; 232: 91–100
27. Harrison, JD Epivatianos, A Bhatia, SN. Role of microliths in the aetiology of chronic submandibular sialadenitis: a clinicopathological investigation of 154 cases. Histopathology 1997; 31: 237–251
28. Harrison, JD Badir, MS. Chronic submandibular sialadenitis: ultrastructure and phosphatase histochemistry. Ultrastruct Pathol 1998; 22: 431–437
29. Antoniades, D Harrison, JD Epivatianos, A Papanayotou, P. Treatment of chronic sialadenitis by intraductal penicillin or saline. J Oral Maxillofac Surg 2004; 62: 431–434
30. Baurmash, HD. Chronic recurrent parotitis: a closer look at its origin, diagnosis and management. J Oral Maxillofac Surg 2004; 62 (8): 1010–1018
31. Scott, J Flower, EA Burns, J. A quantitative study of histological changes in the human parotid gland occurring with adult age. J Oral Pathol 1987; 16: 505–510
32. Jensen, JL Howell, FV Rick, GM Correll, RW. Minor salivary gland calculi: a clinicopathological study of forty-seven new cases. Oral Surg Oral Med Oral Pathol 1979; 47: 44–50
33. Anneroth, G Hansen, LS. Minor salivary gland calculi: a clinical and histopathological study of 49 cases. Int J Oral Surg 1983; 12: 80–89
34. Yamane, GM Scharlock, SE Jain, R. SunderRaj M, Chaudhry AP. Intraoral minor salivary gland sialolithiasis. J Oral Med 1984; 39: 85–90
35. Harrison, JD. Salivary mucoceles. Oral Surg Oral Med Oral Pathol 1975; 39: 268–278
36. Scott, J. Qualitative and quantitative observations on the histology of human labial salivary glands obtained post mortem. J Biol Buccale 1980; 8: 187–200
37. Anneroth, G Isacsson, G Lundquist, PG. The mineral content of salivary calculi: a quantitative microradiographic and diffractometric study. Dentomaxillofac Radiol 1979; 8: 33–41
38. Grases, F Santiago, C Simonet, BM Costa-Bauzá, A. Sialolithiasis: mechanism of calculi formation and etiologic factors. Clin Chim Acta 2003; 334: 131–136
39. Zenk, J Iro, H. [Sialolithiasis and its treatment.] Laryngo-Rhino- Otol 2001; 80 (Suppl 1): 115–136
40. Harrill, JA King, JS Boyce, WH. Structure and composition of salivary calculi. Laryngoscope 1959; 69: 481–492
41. Anneroth, G Eneroth, CM Isacsson, G. The relation of lipids to the mineral component in salivary calculi. J Oral Pathol 1977; 6: 373–381
42. Anneroth, G Eneroth, CM Isacsson, G. Morphology of salivary calculi: the distribution of the inorganic component. J Oral Pathol 1975; 4: 257–265
43. Anneroth, G Eneroth, CM Isacsson, G. Crystalline structure of salivary calculi: a microradiographic and microdiffractometric study. J Oral Pathol 1975; 4: 266–272
44. Anneroth, G Eneroth, CM Isacsson, G Lundquist, PG. Ultrastructure of salivary calculi. Scand J Dent Res 1978; 86: 182–192
45. Lustmann, J Shteyer, A. Salivary calculi: ultrastructural morphology and bacterial etiology. J Dent Res 1981; 60: 1386–1395
46. Isacsson, G Friskopp, J. The morphology of salivary calculi: a scanning electron microscopic study. Acta Odontol Scand 1984; 42: 65–72
47. Teymoortash, A Wollstein, AC Lippert, BM Peldszus, R Werner, JA. Bacteria and pathogenesis of human salivary calculus. Acta Otolaryngol 2002; 122: 210–214
48. Takeda, Y. Crystalloids with calcareous deposition in the parotid gland: one of the possible causes of development of salivary calculi. J Oral Pathol 1986; 15: 459–461
49. Riesco, JM Juanes, JA Díaz-González, MP Blanco, EJ Riesco- López, JM Vázquez, R. Crystalloid architecture of a sialolith in a minor salivary gland. J Oral Pathol Med 1999; 28: 451–455
50. Burstein, LS Boskey, AL Tannenbaum, PJ Posner, AS Mandel, ID. The crystal chemistry of submandibular and parotid salivary gland stones. J Oral Pathol 1979; 8: 284–291
51. Tanaka, N Ichinose, S Adachi, Y Mimura, M Kimijima, Y. Ultrastructural analysis of salivary calculus in combination with X-ray microanalysis. Med Electron Microsc 2003; 36: 120–126
52. Teymoortash, A Buck, P Jepsen, H Werner, JA. Sialolith crystals localized intraglandularly and in the Wharton’s duct of the human submandibular gland: an X-ray diffraction analysis. Arch Oral Biol 2003; 48: 233–236
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


