Traumatic injuries of the facial nerve are uncommon compared with other causes of facial nerve dysfunction, such as tumors, cerebral ischemia, and idiopathic nerve palsy. Facial nerve damage caused by head or neck trauma may be associated with life-threatening injuries, and therapy focuses on life-saving measures and stabilization of the patient’s general condition. Although a traumatic lesion of the facial nerve may not appear to be of primary importance in the emergency room, permanent facial paralysis caused by traumatic injury can severely affect the patient’s life thereafter. Loss of facial movement and insufficient eye closure are burdensome, and the social consequences of disfigurement and loss of facial expression are particularly distressing. The sequelae are likely to impair the patient’s personal relationships and social life in a profound manner.
Adequate management of the traumatic injuries of the facial nerve requires a thorough knowledge of the complex anatomy of the nerve, sophisticated diagnostic means, and advanced surgical skills in microsurgical nerve repair. The surgeons must provide the conditions for successful recovery or repair of traumatic nerve damage. This chapter discusses initial presentation, diagnostic measures, and timing and techniques of nerve repair.
Epidemiology
Traumatic damage to the facial nerve can be indirect or direct. Indirect damage may be encountered after intracerebral bleeding. Because intracerebral lesions of the facial nerve nucleus or its fascicles are unlikely to be directly repaired surgically, they are not considered in this chapter.
Direct trauma to the nerve can occur along its intratemporal and extratemporal routes. The most common site of direct traumatic lesions is the intratemporal route, and trauma usually is caused by temporal bone fractures (86%). However, only 7% of temporal fractures involve facial nerve damage, which indicates that direct facial nerve injuries in head and neck trauma are uncommon. The second most frequent cause of traumatic damage to the facial nerve is gunshot wounds to the temporal bone, although only 8% of all gunshot wounds in the head and neck are associated with facial nerve injury. Damage to the nerve on the extratemporal route occurs even less frequently. Blunt injuries, stab wounds, severely dislocated mandibular fractures, gunshot wounds, and birth trauma can account for facial paralysis. In gunshot wounds, traumatic damage may occur through blast injury, even if the trunk or the large branches have not been directly severed.
Obstetrical reasons for facial nerve lesions are rare, occurring in approximately 0.07% of deliveries, but facial nerve damage is nevertheless the second or third most common injury caused by obstetrical trauma. Only 6.3% of facial nerve disorders result from direct traumatic injuries. Occasionally, late damage to the facial nerve may be associated with posttraumatic vascular malformations.
State-of-the-Art Management
Diagnosis of acute facial nerve damage due to trauma is commonly impaired by the general anaesthetic administered in the emergency room. Facial nerve damage often must be assumed from the location and extent of soft tissue wounds. However, even if direct damage to parts of the facial nerve appears to be very likely from clinical inspection, not every traumatic lesion of the facial nerve is inevitably followed by complete paralysis of the facial muscles. Even in extensive lacerations of facial soft tissues, nerve function may not be damaged, or after initial weakness, a level of adequate function may be recovered ( Fig. 21-1 ).
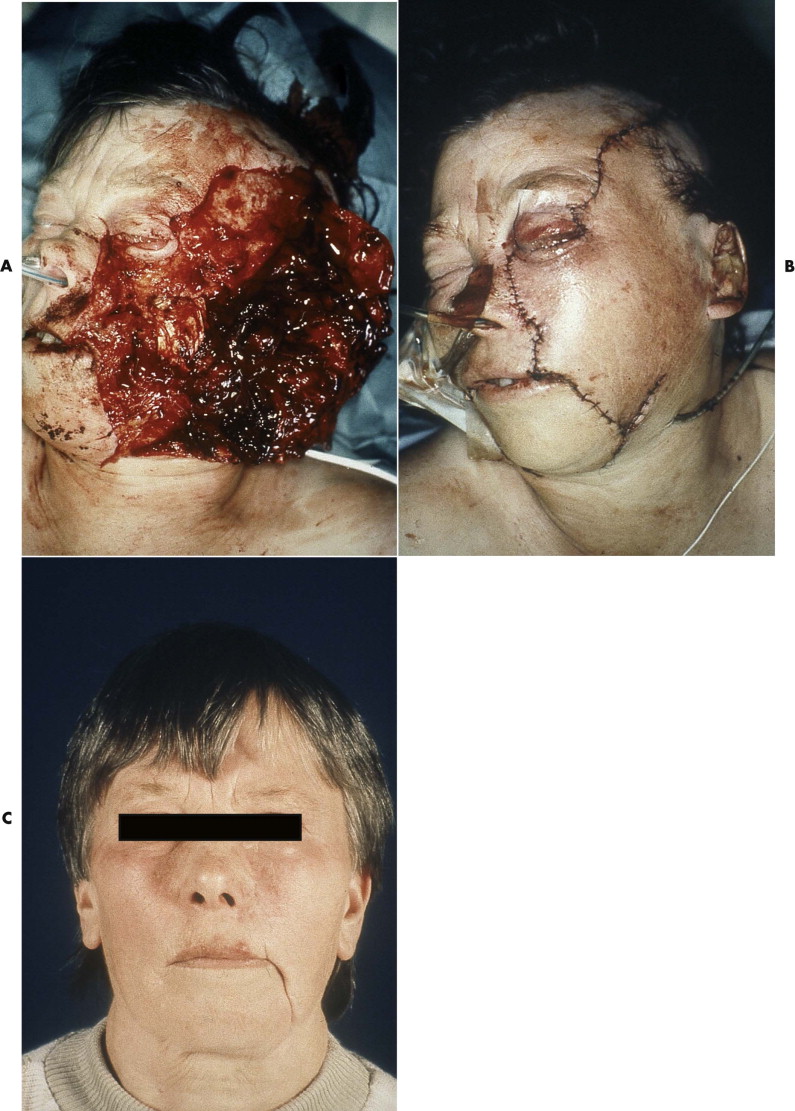
Several factors influence treatment outcomes. The nerve is completely embedded in soft tissue and has considerable longitudinal elasticity, which accounts for its amazing resistance against mechanical damage, even during direct impact. An extensive system of anastomosis between the trunks of the nerve supplies every region of the facial muscles except the mandibular branch. Multiple nerve supplies, with mutual exchange between fibers of different branches, usually exist between the zygomatic and buccal branches. Isolated damage to one of these branches commonly does not result in a paresis of the corresponding facial muscles. However, clinical evidence of nerve dysfunction based on weakness of the facial muscles is not necessarily associated with severing of, or irreversible damage to, the corresponding nerve fibers.
Nerve damage has been classified into three categories: neurapraxia, axonotmesis, and neurotmesis. Although these categories refer to structural alterations of the nerve at a microscopic level, they are directly related to the patient’s prognosis and clinical outcome.
Neurapraxia is the simplest type of injury to the nerve, and its clinical manifestation is only temporary. The axons of the nerve remain undamaged, but the myelin sheath sustains injury, such as from compression or hyperextension of the nerve. Resulting edema of the myelin sheath causes temporary dysfunction, from which the nerve recovers within days to weeks.
In axonotmesis, continuity of the nerve is preserved, but damage is more severe due to prolonged compression or a localized ischemic lesion of the nerve. The myelin sheath and the axons are affected. Both components degenerate, but regeneration of axons is guided by the fibrous structures of the nerve, which remain intact. In electromyographic recordings, axonotmesis is characterized by degenerative reactions, retardation of muscle twitching, and complete interruption of nerve conduction. However, because the continuity of the fibrous framework of the nerve has been maintained along the entire length of the nerve, regeneration can proceed to the distal end of the nerve. The axons and the myelin sheath undergo wallerian degeneration, after which their debris is digested by Schwann cells and invading macrophages. Concurrently, Schwann cells proliferate within the basal membrane and arrange themselves into chains known as Hanken-Büngner bands. Regeneration of axons then commences proximal to the site of damage and follows the Hanken-Büngner bands to the periphery. Regeneration of the normal nerve fiber pattern is possible, resulting in more or less complete functional restoration.
In neurotmesis, the nerve is completely severed, and its ends are separated by retraction due to the longitudinal elasticity of the nerve. Clinically and electromyographically, axonotmesis and neurotmesis are identical. The morphological alterations during degeneration of axons and myelin sheath are likewise the same. However, spontaneous regeneration in the peripheral nerve segment is impossible because it depends on bridging the gap between the two retracted nerve ends. Surgical intervention is required to establish continuity of the nerve by direct suturing or grafting.
State-of-the-Art Management
Diagnosis of acute facial nerve damage due to trauma is commonly impaired by the general anaesthetic administered in the emergency room. Facial nerve damage often must be assumed from the location and extent of soft tissue wounds. However, even if direct damage to parts of the facial nerve appears to be very likely from clinical inspection, not every traumatic lesion of the facial nerve is inevitably followed by complete paralysis of the facial muscles. Even in extensive lacerations of facial soft tissues, nerve function may not be damaged, or after initial weakness, a level of adequate function may be recovered ( Fig. 21-1 ).
Several factors influence treatment outcomes. The nerve is completely embedded in soft tissue and has considerable longitudinal elasticity, which accounts for its amazing resistance against mechanical damage, even during direct impact. An extensive system of anastomosis between the trunks of the nerve supplies every region of the facial muscles except the mandibular branch. Multiple nerve supplies, with mutual exchange between fibers of different branches, usually exist between the zygomatic and buccal branches. Isolated damage to one of these branches commonly does not result in a paresis of the corresponding facial muscles. However, clinical evidence of nerve dysfunction based on weakness of the facial muscles is not necessarily associated with severing of, or irreversible damage to, the corresponding nerve fibers.
Nerve damage has been classified into three categories: neurapraxia, axonotmesis, and neurotmesis. Although these categories refer to structural alterations of the nerve at a microscopic level, they are directly related to the patient’s prognosis and clinical outcome.
Neurapraxia is the simplest type of injury to the nerve, and its clinical manifestation is only temporary. The axons of the nerve remain undamaged, but the myelin sheath sustains injury, such as from compression or hyperextension of the nerve. Resulting edema of the myelin sheath causes temporary dysfunction, from which the nerve recovers within days to weeks.
In axonotmesis, continuity of the nerve is preserved, but damage is more severe due to prolonged compression or a localized ischemic lesion of the nerve. The myelin sheath and the axons are affected. Both components degenerate, but regeneration of axons is guided by the fibrous structures of the nerve, which remain intact. In electromyographic recordings, axonotmesis is characterized by degenerative reactions, retardation of muscle twitching, and complete interruption of nerve conduction. However, because the continuity of the fibrous framework of the nerve has been maintained along the entire length of the nerve, regeneration can proceed to the distal end of the nerve. The axons and the myelin sheath undergo wallerian degeneration, after which their debris is digested by Schwann cells and invading macrophages. Concurrently, Schwann cells proliferate within the basal membrane and arrange themselves into chains known as Hanken-Büngner bands. Regeneration of axons then commences proximal to the site of damage and follows the Hanken-Büngner bands to the periphery. Regeneration of the normal nerve fiber pattern is possible, resulting in more or less complete functional restoration.
In neurotmesis, the nerve is completely severed, and its ends are separated by retraction due to the longitudinal elasticity of the nerve. Clinically and electromyographically, axonotmesis and neurotmesis are identical. The morphological alterations during degeneration of axons and myelin sheath are likewise the same. However, spontaneous regeneration in the peripheral nerve segment is impossible because it depends on bridging the gap between the two retracted nerve ends. Surgical intervention is required to establish continuity of the nerve by direct suturing or grafting.
Diagnosis and Timing of Treatment
In conscious and cooperative patients, function of the facial muscles is easily tested by asking the patient to sequentially activate the frontal, periorbital, buccal, and perioral muscles. However, clinical signs of facial nerve palsy also can manifest with some delay in mastoid bone fractures.
In injuries with suspected intratemporal damage to the nerve, diagnostic imaging is required. In temporal bone fractures, computed tomography is preferable to plain radiography, because only 20% to 30% of temporal fractures can be visualized by the latter technique. Magnetic resonance imaging (MRI) provides additional information about facial nerve damage by showing abnormal nerve enhancement, particularly in distal intrameatal nerve segments. MRI enhancement by gadolinium-diethyl-triamine-pentaacetic acid (Gd-DTPA) produces more informative studies for the evaluation of facial nerve pathology and can help to define nerve lesions more accurately. Contrast-enhanced scans can identify clinically silent traumatic damage in the temporal bone. Electrodiagnostic tests such as evoked electromyography or electroneuronography play an important role in the objective assessment of facial nerve damage and provide indications for decision making with respect to surgical intervention. However, most electrodiagnostic tests are focused on examination of the nerve distal to the stylomastoid foramen and cannot evaluate the nerve across the injury site in intratemporal lesions. Pathological findings are obtained on electrodiagnostic nerve testing only if wallerian degeneration of the axons distal to the damaged site has occurred. These diagnostic tools are useful only for late assessment of posttraumatic facial nerve damage.
To provide earlier information about the severity of facial nerve lesions, transcranial magnetic stimulation of the facial nerve after nontraumatic damage has been used to evaluate patients with Bell’s palsy. This technique has also been applied successfully in the immediate examination of traumatic facial nerve lesions in an experimental setting. However, contradictory results in clinical and experimental studies preclude recommendation of its routine clinical use at this time.
If deep lacerations of facial soft tissues in the buccal and particularly the preauricular area indicate damage on the extratemporal route of the nerve and there is clinical evidence of paresis, meticulous inspection and identification of severed nerve ends is often the only way to positively establish the presence of nerve damage. The functional sequelae of evident damage to individual nerve branches are difficult to predict because of the reasons mentioned earlier, and primary nerve reconstruction after identification of nerve ends is not always indicated. From the experience with crossfacial nerve grafts for facial reanimation, it is known that 50% of facial nerve fibers are sufficient to provide satisfactory muscle tonus at rest and symmetrical movement under voluntary function. Primary repair is recommended only if there is morphological proof that the trunk of the facial nerve itself has been severed. Only in this case should further dissection of the nerve segments be performed. However, if primary repair is not possible because of soft tissue swelling, bleeding, lack of operation time, or serious injury, repair can be delayed. Reconstruction after 3 to 4 weeks may be preferable in many cases because the patient’s condition has become stable and soft tissue swelling has gone down. For the severed nerve ends to be identified in the secondary approach, they must be marked during primary care with non-resorbable atraumatic marker sutures that are fixed to the skin.
If the patient has an extratemporal traumatic facial nerve lesion and the surgeon is uncertain whether the injury is only minor (i.e., neurapraxia) or the nerve has been completely severed, no more than 3 months should elapse before possible repair. During this time, the nerve function must be monitored electromyographically, and a decision about whether to perform a reconstruction must be made. If no signs of regeneration are seen during this period, revision and reconstruction should no longer be delayed. Physical therapy such as electrostimulation of the facial muscles and massage should be administered in the meantime.
The interval before secondary nerve repair is limited by the progressive atrophy of the facial muscles on the paretic side of the face. If reconstruction of the nerve is considered, it must be performed at least within the first year postoperatively, because after this period, muscle function, even with reinnervated facial nerve fibers, will be insufficient to accomplish satisfactory movement of the face on the paretic side. Beyond this interval, reanimation of the paralyzed face must be performed by revascularized transfer of neuromuscular segments. In such procedures, a vascularized segment from the gracilis muscle or the latissimus dorsi muscle with a branch of the supplying nerve is transferred to the face to augment the atrophic facial muscles and allow for innervated function of the transferred muscle.
Surgical Repair
Surgical intervention for repair of traumatic damage to the facial nerve depends on the location of the nerve injury. For intratemporal lesions, decompression surgery of the nerve in the osseous canal through the temporal bone must be considered. For extratemporal lesions, a decision must be made about microsurgical repair of the nerve. Although the former procedure is more a domain of otolaryngology or neurosurgery, the maxillofacial surgeon is involved in the latter.
Microsurgical repair of the extratemporal portion of the facial nerve requires a high standard of technical equipment and surgical training. A surgical microscope with continuously adjustable magnification between 2× and 40× and foot control should be available. Suture material with a 25 µm diameter (10-0) is recommended, and forceps with smooth, nonserrated ends are preferable to avoid trauma to the delicate perineural tissue.
Nerve Suturing
Basic surgical techniques for repair of the extratemporal part of the facial nerve have been contributed by Conley and Miehlke. Before the use of the microscope for surgical nerve repair, nerve suturing was commonly performed by suturing the epineurium. This was often unsuccessful, mainly because of inadequate preparation of the cross-sectional surface of the nerve ends and insufficient adaptation of the individual fascicles. Surgery has dramatically improved since the introduction of microscopic techniques, which enabled atraumatic handling and precise adaptation of the nerve ends and individual fascicles. The poor results obtained with epineural suturing in the earlier era were initially attributed to the epineural location of the suture, and new concepts of perineural suturing with adaptation of individual fascicles were developed. Numerous studies have now shown that both techniques can achieve similar results as long as they are performed with the same accuracy and atraumatic handling of the nerve ends and fascicles.
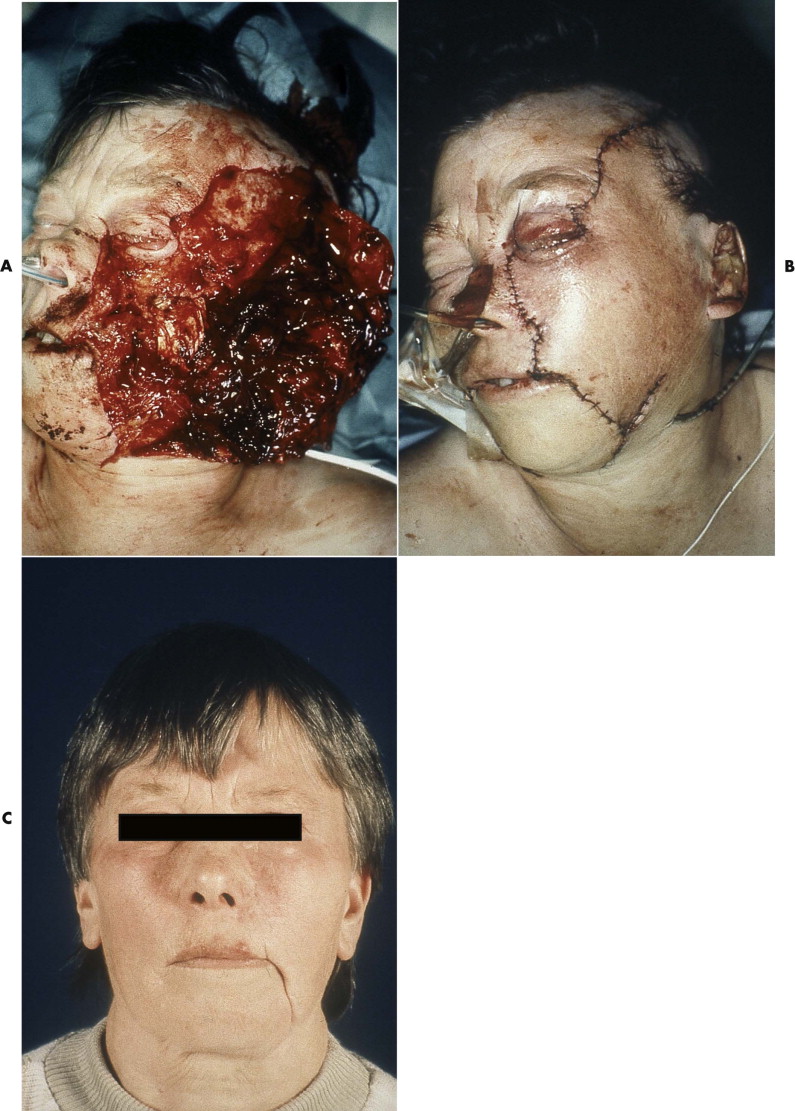
The differential indication for epineural versus perineural suturing depends on the fascicular structure of the nerve. The three patterns of fascicles are monofascicular, oligofascicular (fewer than five fascicles), and polyfascicular structure ( Fig. 21-2 ). Epineural suturing is considered to be appropriate in monofascicular and oligofascicular nerve ends, and perineural sutures are required only in polyfascicular nerves. Because the facial nerve has a monofascicular or oligofascicular structure at the nerve trunk and its first divisions into peripheral branches, epineural suturing is adequate in most areas of the nerve.
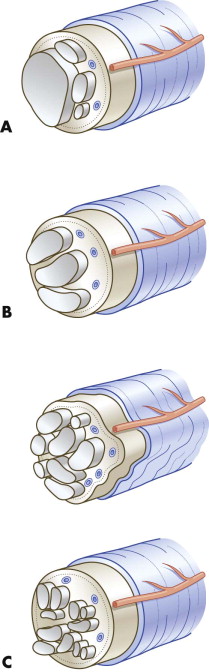
The aim and strategy of microsurgical intervention are defined by the timing of the surgery and the degree of damage to the nerve. In primary repair and any other microsurgical repair procedure, the nerve ends are dissected free of epineural connective tissue ( Fig. 21-3A ), because the greatest hazard for axon proliferation from the central nerve end into the peripheral segment is the proliferation of connective tissue at the site of suturing. Intervening connective tissue can prevent axons from further elongation into the distal segment or can turn down already regenerated axons. Possible factors that account for this fibrosis are traumatic dissection of the nerve during preparation for suturing and suturing of the nerve ends while they are under longitudinal tension. Tension-free suturing of the nerve ends and atraumatic dissection are mandatory.
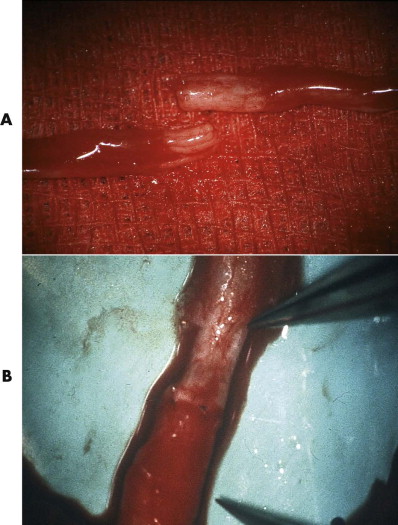
Because the epineural fibroblasts proliferate more rapidly than the fibroblasts and Schwann cells of the endoneural space, the epineural tissue should be removed from the nerve ends to reduce the proportion of connective tissue in nerve cross-section and to allow for more precise adaptation of the fascicles (see Fig. 21-3B ). However, removal of the epineurium in facial nerve repair is feasible only at the trunk, because the epineurium becomes very thin on the more peripheral branches and is hardly discernible from the remaining parts.
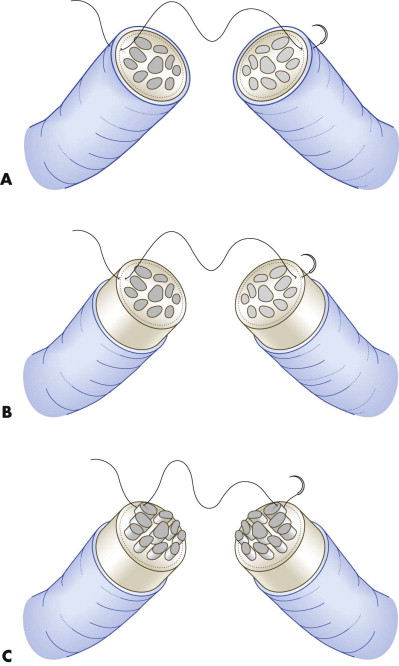
Before the nerve ends are sutured in primary repair after trauma, they are dissected free of their surrounding tissue and trimmed with serrated scissors until the cut ends look completely undamaged. Subsequently, the epineurium is opened and removed on both ends for a length of 5 to 10 mm with the scissors at a microscopic magnification of 10×. This reveals the location and structure of fascicles at the cross-section of the nerve. After trimming, axons tend to prolapse due to endoneural pressure and longitudinal retraction of the perineural tissue. These protruding axons should be removed to gain a smooth cross-sectional area that facilitates adaptation and suturing of the nerve ends. Protruding axons are grasped with the forceps, pulled forward, and cut with serrated scissors at the level of the epineural or perineural annulus. Suturing can be performed by epineural, perineural, or interfascicular suturing ( Fig. 21-4 ).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


