Anatomy of the Masticatory System
A rational clinical examination of the masticatory system requires a sound basic knowledge of the anatomy. As will become clear later in the discussion of clinical examination procedures, the foundation of manual functional analysis is a good knowledge of the functional anatomy. In this chapter the individual anatomical structures will be described in a sequence corresponding to the later examination steps and separated according to their physiology and stages of adaptation and compensation. Knowledge of the different progressive and regressive tissue reactions is not only relevant to the diagnostic interpretation of the findings, but it also decisively influences the treatment strategy. The division into physiological, compensated, and adapted masticatory systems is necessary not only for diagnostic purposes but, more importantly, for the determination of what treatment goals are attainable for the individual.
The human jaw articulation is a so-called secondary joint (Gaupp 1911) because it developed separately and not as a modification of a primary joint (Dabelow 1928). The essential morphogenetic events in the formation of the joints of the jaw occur between the seventh and twentieth embryonic weeks (Baume 1962, Furstman 1963. Moffet 1957, Baume and Holz 1970, Blackwood et al. 1976. Keith 1982, Perry et al. 1985, Burdi 1992, Klesper and Koebke 1993. Valenza et al. 1993, Bach-Petersen et al. 1994. Ögütcen-Toller and juniper 1994, Bontschev 1996, Rodriguez-Vazquez et al. 1997). The critical period for the appearance of malformations in the joints of the jaw is reported differently in different studies. According to Van der Linden et al. (1987) it is between the seventh and eleventh weeks, according to Furstman (1963) between the eighth and twelfth weeks, and according to Moore and Lavelle (1974), between the tenth and twelfth weeks.
Formation of the bony mandible begins in weeks 6-7 lateral to Meckel’s cartilage in both halves of the face. A double anlage of Meckel’s cartilage is extremely rare (Rodriguez-Vazquez et al. 1997). Its effect on embryonic development is unknown. By about the twelfth week the two palatal processes have united at the midline to complete the separation of the oral and nasal cavities. At the same time, bony anlagen of the maxilla form in the region of the future infraorbital foramina. These spread rapidly in a horizontal direction and progressively fill the space between the oral cavity and the eyes. When the crown-rump length (CRL) is approximately 76 mm (weeks 10-12), the anlagen of the maxillary bone, the zygomatic bone, and the temporal bone come into contact with one another. Ossification of the base of the cranium and of the facial portion of the skull follows in a strict, genetically determined sequence (Bach-Petersen et al. 1994). First to ossify is the mandible, followed by the maxilla, medial alar process of the sphenoid bone, frontal bone, zygomatic bone, zygomatic arch, squamous part of the occipital bone, greater wing of the sphenoid bone, tympanic bone, condyles of the occipital bone, lesser wing of the sphenoid bone, and finally the dorsolateral portion of the sphenoid bone.
In an embryo with a CRL of approximately 53 mm the coronoid process and the condylar process can already be clearly distinguished from one another. The biconcave form of the articular disk becomes apparent at a CRL of 83 mm. In histological preparations, fibers of the pterygoid muscle can also be seen streaming in quite early (Radlanski et al. 1994). At this stage the superior belly of the lateral pterygoid muscle inserts at the middle and central third of the disk and the lower belly inserts at the condyle (Merida-Velasco et al. 1993). At a CRL of 95 mm all structures of the temporomandibular joint can be clearly identified and thereafter undergo no essential change other than an increase in size (Bontschev 1996).
Embryology of the Temporomandibular Joint and the Muscles of Mastication
During the development of the temporomandibular joint the articular fossa is the first structure to become recognizable. This occurs during weeks 7-8 (Burdi 1992). It First appears as a concentration of mesenchymal cells over an area of tissue that later differentiates into disk and capsule. Between the tenth and eleventh weeks the fossa begins to ossify. Development of the cortical layer and the bony trabeculae is more rapid in the fossa than in the condyle. The fossa develops first as a protrusion on the original site of the zygomatic arch and grows in a medial-anterior direction (Lieck 1997). At the same time the articular eminence begins to develop. The condyle, at first cartilaginous, develops between the tenth and eleventh weeks from an accumulation of mesenchymal cells lateral to Meckel’s cartilage (Burdi 1992). Enchondral ossification progresses apically, creating a bony fusion with the body of the mandible. After the fifteenth week the chondrocytes have differentiated enough so that the cartilage already exhibits the typical postnatal organization of structure (Perry et al. 1985), and from the twentieth prenatal week onward only the superficial portion of the process consists of cartilage.
Joint development
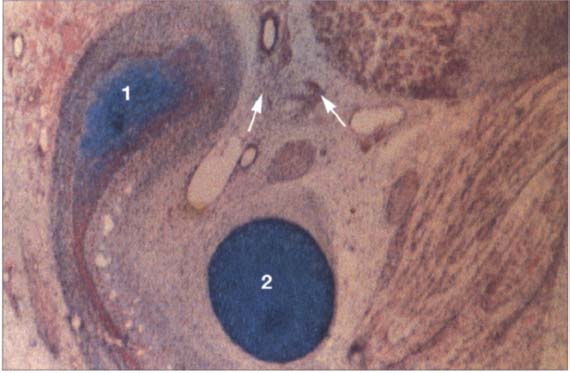
18 Tenth week
A histological section in the frontal plane showing the condylar process (1) and Meckel’s cartilage (2) at the tenth week of embryonic development. The condylar process is rounded over and surrounded by a layer of especially dense mesenchyme (arrows). It lies lateral to Meckel’s cartilage. The fast-growing dorsocranial portion of the accumulation of cartilage cells creates the distinctive shape of the condyle.
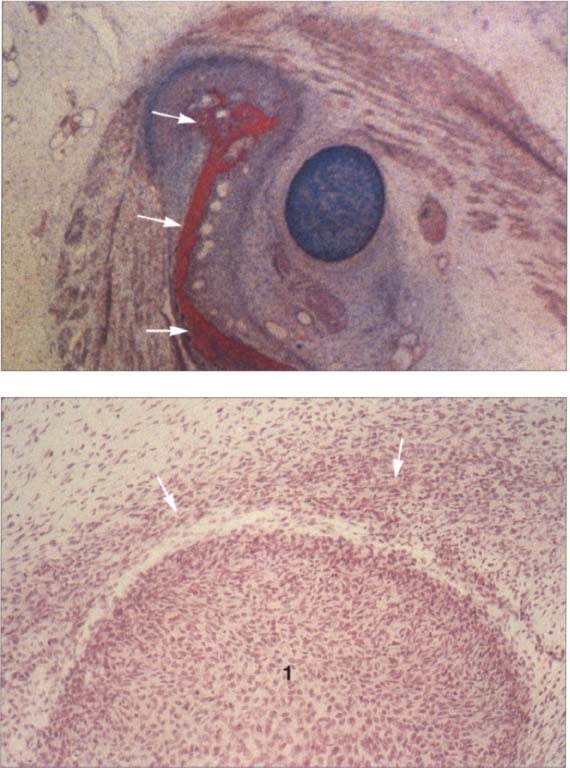
19 Eleventh week
Above: A human temporomandibular joint in the frontal plane at the eleventh week of development. This represents the same area shown in Figure 19 only 10 days further along. The condylar process is beginning to ossify (arrows). At this time the swallowing reflex is also developing and is accompanied by the formation of secondary cartilage (n the temporomandibular joint (Lakars 1995). Contributed by R. Wurgaft Dreiman Below: Sagittal section of a temporomandibular joint at the same stage of development. Above the condyle (1) is a distinct concentration of mesenchymal cells (arrows). At its inferior region the mesenchymal thickening is already beginning to detach from the condyle as the lower joint space forms. During this time the first collagen fibers of the disk become visible and increase greatly in number until the twelfth week. Contributed by R.j. Radlanski
The articular disk can first be identified after 7.5 weeks in utero as a horizontal concentration of mesenchymal cells (Burdi 1992). Between weeks 19 and 20 its typical fibrocartilaginous structure is already evident.
The joint capsule first appears between weeks 9 and 11 as thin striations around the future joint region (Burdi 1992). After 17 weeks the capsule is clearly demarcated, and after 26 weeks all of its cellular and synovial parts are completely differentiated.
In weeks 9-10 the lateral pterygoid muscle is recognizable with its superior head inserting on the disk and capsule and its inferior head inserting on the condyle. Fibers of the masseter and temporal muscles also insert on the disk (Merida Velasco et al. 1993).
During the tenth week the first blood vessels become organized around the joint. The disk has small blood vessels only at its periphery and is itself avascular (Valenza et al. 1993). Branches of the trigeminal and auriculotemporal nerves are clearly visible in the twelfth week (Furstman 1963). The numerous nerve endings that can still be seen in the disk in the twentieth week diminish rapidly so that after birth the disk is no longer innervated (Ramieri et al. 1996).
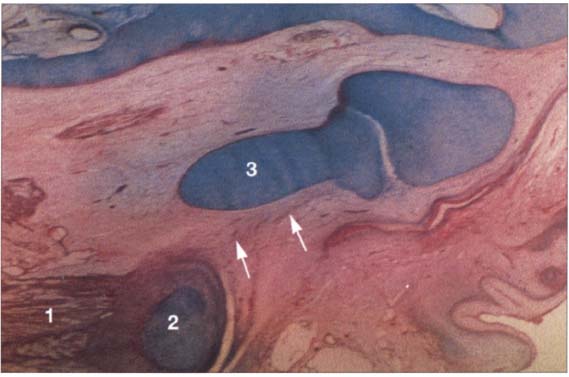
20 Fourteenth week
Sagittal section of a human disk-condyle complex. A distinct joint space has now formed between the condyle (1) and the disk (2). Above the disk the temporal blastema begins to split away to form the upper joint space (arrows). The cartilage of the condyle is increasingly replaced by bone the original cartilage remain in the neck of the condyle until past puberty.
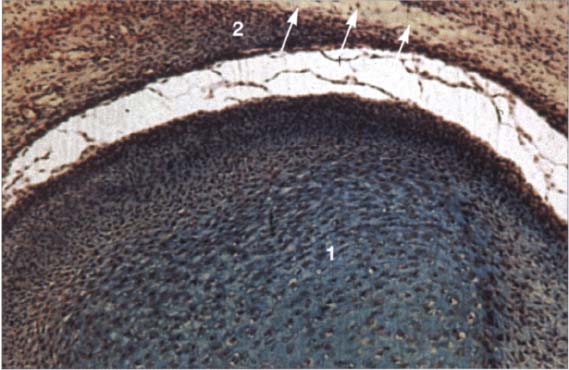
21 Sixteenth week
Horizontal section of a human temporomandibular joint during the sixteenth week of embryonic development. Insertion of the lateral pterygoid muscle (1) onto the condyle (2) can be clearly identified. In agreement with reports in the literature (Ögütcen-Toller and juniper 1994. Ögütcen-Toller 1995), the discomaleolar ligament (arrows) runs from the joint capsule through the tympanosquamosal fissure to the malleus (3) as an extension of the muscle.
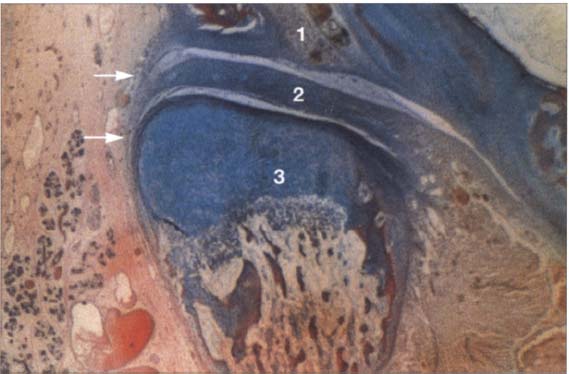
22 Eighteenth week
Frontal section through a human temporomandibular joint in the eighteenth week of embryonic development. The fossa (1), disk (2) and condyle (3) are completely developed and from now on will experience joint capsule (arrows) can also be clearly identified. The cartilaginous condyle will ossify further. Distribution of cartilage at this stage indicates that future growth will be primarily in the laterosuperior direction. Contributed by R. Wurgaft Dreiman
Development of the Upper and Lower Joint Spaces
The upper and lower joint spaces arise through the formation of multiple small splits in the dense mesenchyme from which the condyle, disk, and fossa arose previously.
The lower joint space appears first at about the tenth week (50-65 mm CRL), but later the upper joint space overtakes it in its development (Burdi 1992). At first the space is extensively compartmentalized, and it is only later that the individual cavities merge (Bontschev 1996). The lower joint space lies close to the embryonic condyle.
The upper joint space appears after about the twelfth week (60-70 mm CRL) and spreads posteriorly and medially over Meckel’s cartilage with its contour corresponding to that of the future fossa. After week 13 the lower joint space is already well formed as the upper joint space continues to take shape. From its beginning, the upper joint space has fewer individual islands of space and grows more rapidly than the lower joint space. After week 14 both joint spaces are completely formed. During weeks 16-22 the lumens of the chambers become adapted to the contours of the surrounding rounding bone. The fibrocartilaginous articular disk develops from the concentrated mesenchyme between the two joint spaces. The articular disk is not visible until the CRL is 70 mm. Even before formation of the joint spaces the disk is already thinner at its center than at the periphery and this leads to its final biconcave form (Bontschew 1996). The peripheral portions are not sharply demarcated from the surrounding loose mesenchyme. In fetuses with a CRL of 240 mm, the mesenchymal tissue changes into dense fibrous connective tissue. At this stage the peripheral region has a greater blood supply than the central region. According to Moffet (1957), compression of the disk between the temporal bone and the condyle results in an avascular central zone. At the beginning of its development the disk lies closer to the condylar process than to the future fossa. At this stage there is still a layer of loose mesenchyme between the temporal bone and the upper joint space. It is only after a CRL of 95 mm has been reached that the condylar process and the fossa become closer and the mesenchymal layer disappears.
Joint development
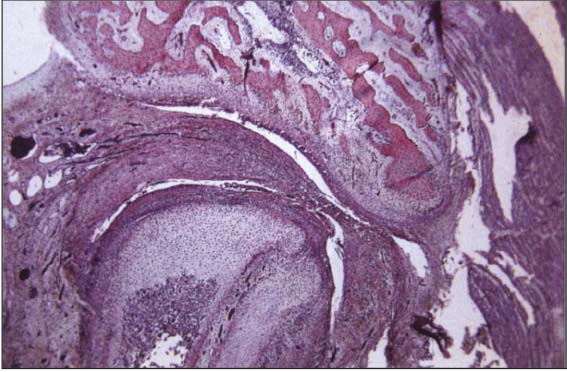
23 Twenty-sixth week
Completely formed human temporomandibular joint with physiolgical lower and upper joint spaces. Trabecula-like structures can be identified in both joint spaces where the disk has not yet separated completely from the temporal and condylar portions. At present it has not been conclusively determined whether or not this type of incomplete separation could be one cause of disk adhesions.
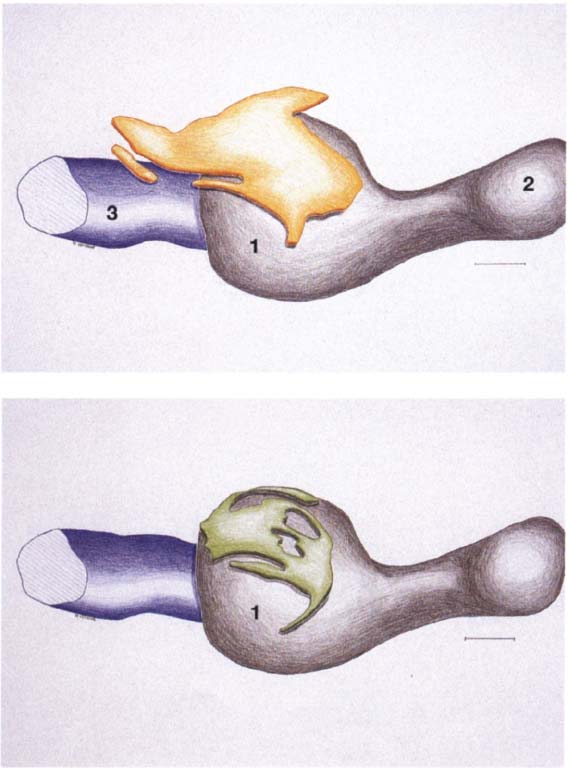
24 Development of the joint spaces
Above: Three-dimensional reconstruction from a series of histological sections of the developing joint space (yellow) of a right temporomandibular joint. In the center of the picture is the condyle (1); to the right of it lies the coronoid process (2). To the left behind the condyle is Meckel’s cartilage (3). The upper joint space arises approximately 2 weeks after the lower. Below: Three-dimensional reconstruction of the lower joint space (green) of the S3me joint. Initially the mesenchyme in the condylar region (1) is still uniformly structured, but in weeks 10-12 it begins to tear in several places mesial and distal to the condyle. The resulting clefts run together to form the lower joint space. A region of concentrated mesenchyme remains between the two joint spaces, from which the fibrocartilaginous articular disk is later formed. Contributed by R. J. Radianski (Figs. 23–25)
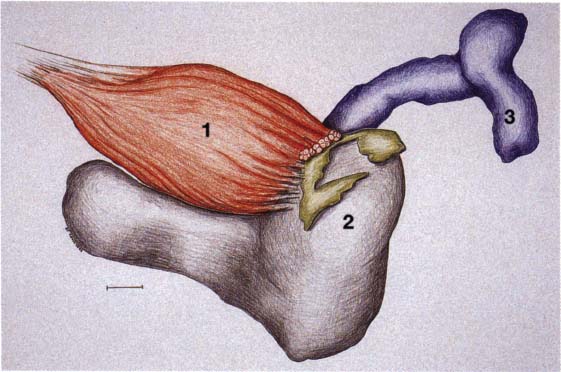
25 Development of the lateral pterygoid muscle
Three-dimensional representation of the insertion of the lateral pterygoid muscle (1) onto a left temporomandibular joint. As the muscle develops from the eleventh week, its upper belly attaches to the condyle, capsule, and disk while its lower belly attaches only to the condyle (2). At no time during development do the fibers of the lateral pterygoid muscle make direct contact with Meckel’s cartilage (Ögütcen-Toller and juniper 1994).
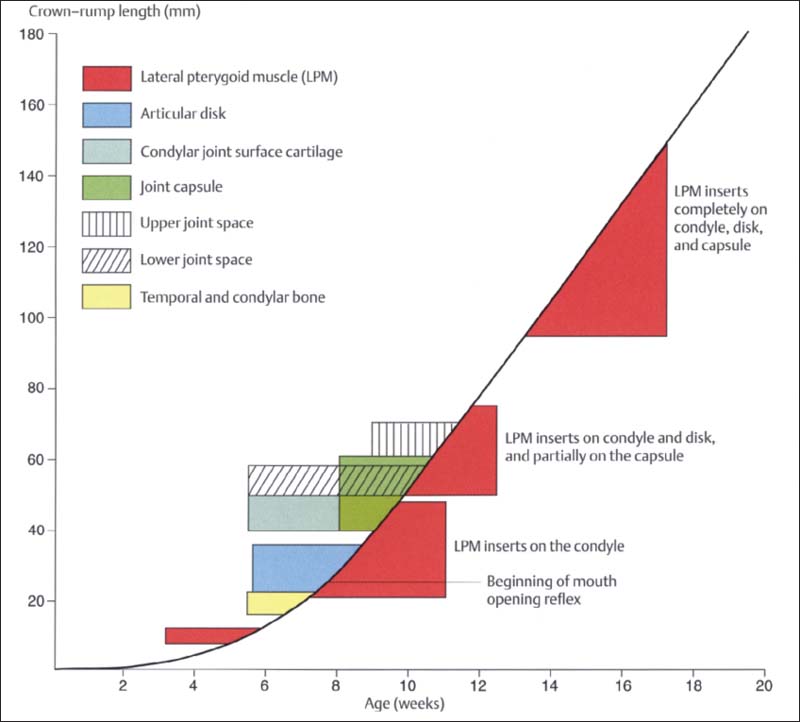
26 Development of the human temporomandibular joint
Graphic representation (modified from van der Linden et al. 1987) of prenatal development of the human temporomandibular joint showing its relationship to the CRL and age. First to form are the bony structures and the disk. Development of the joint capsule is accompanied by development of the upper and lower joint spaces. It is most interesting that prenatal mandibular movements can be observed as early as weeks 7-8 (Hooker 1954, Humphrey 1968), even though most of the joint structures and even the muscle insertions do not develop until a few weeks later. It is assumed that the movements are made possible by the primary jaw joint between Meckel’s cartilage and malleus-incus (Burdi 1992).
Glenoid Fossa and Articular Protuberance
The temporal portion of the joint can be divided into four functional parts from posterior to anterior: postglenoidal process, glenoid fossa, articular protuberance, and apex of the eminence. The inclination of the protuberance to the occlusal plane varies with age and function (Kazanjian 1940), but is 90% determined at the age of 10 years (Nickel et al. 1988). Three fissures can be found at the transition to the tympanic plate of the temporal bone: the squamotympanic, petrotympanic, and petrosquamous fissures (Fig. 28). In patients with disk displacement, these fissures are frequently ossified (Bumann et al. 1991). Under physiological conditions the only parts of the temporal portion of the joint that are covered with secondary cartilage are the protuberance and the eminence (Fig. 31). Secondary cartilage is formed only when there is functional loading. Before the fourth postnatal year stimulation of the cells of the perioseum leads to the formation of secondary cartilage (Hall 1979, Thorogood 1979, Nickel et al. 1997J. With no persisting functional load the chondrocytes of the condyle would differentiate into osteoblasts (Kantomaa and Hall 1991).
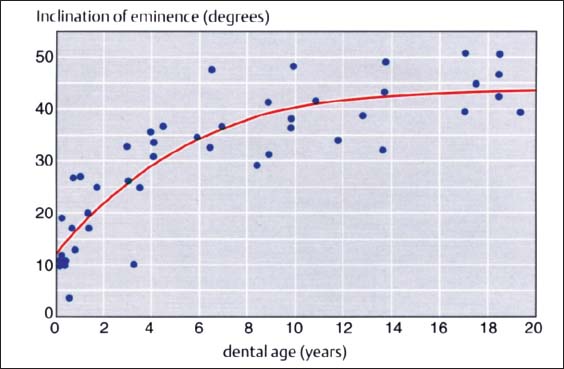
27 Inclination of the articular protuberance to the occlusal plane
This graph (adapted from that of Nickel et al. 1988) indicates the inclination of the posterior slope of the eminence (articular protuberance) in relation to the occlusal plane. Accordingly, at the age of 3 years the eminence has reached 50% of its final shape (Nickel et al. 1997). Between the tenth and twentieth year there is a difference of only 5°. The study material originates from the osteological collection of Hamman-Todd and Johns Hopkins. Cleveland Museum of Natural History.
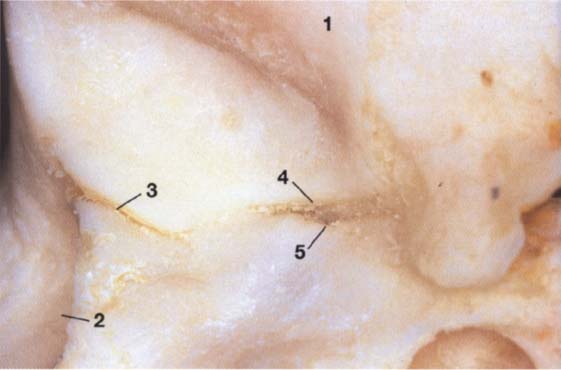
28 Joint region of the temporal bone
Inferior view of the temporal portion of a defleshed temporomandibular joint. Near the upper border of the picture is the articular eminence (1) and at the far left is the external auditory meatus (2). In the posterior portion of the fossa the squamotympanic fissure (3) is found laterally, and the petrosquamous (4) and petrotympanic (5) fissures are found medially. Both the superior stratum of the bilaminar zone and the posterior portion of the joint capsule, and sometimes also the fascia of the parotid gland can insert into these fissures.
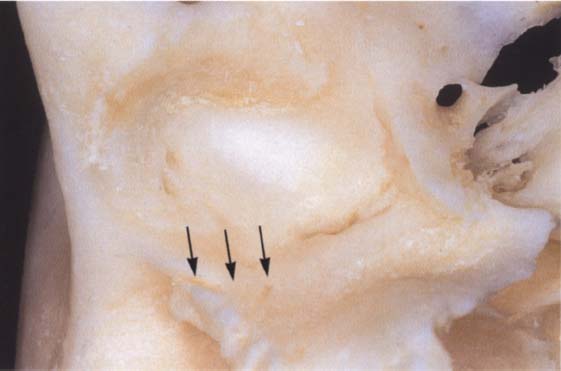
29 Ossification of the fissures and disk displacement
Inferior view of a temporal bone with partially ossified fissures. The lateral half of the squamotympanic fissure is completely ossified (arrows). The superior stratum of the bilaminar zone can now insert only into the periosteum in this region. It has been shown that these fissures are ossified in more than 95% of patients with disk displacement, whereas in joints without disk displacement normal fissure formation prevails (Bumann et al. 1991).
However, the maturation process of these cells is delayed by functional demands (Kantomaa and Hall 1988). Loading reduces the intracellular concentration of cyclic adenosine monophosphate (cAMP). This increases the rate of mitosis and suppresses the ossification process relative to the proliferation of cartilage (Copray et al. 1985). Furthermore, the proteoglycane content of cartilage correlates with its ability to withstand compressive loads (Mow et al. 1992).
The hypothesis that structures of the temporomandibular joint are subjected to compressive loads during function has been around for many decades and is supported by a number of experimental studies (Hylander 1975, Hinton 1981, Taylor 1986, Faulkner et al. 1987, Boyd et al. 1990, Mills et al. 1994a). Studies using finite element analysis (FEA) also verify that during function, temporomandibular joint structures are subject to variable loads depending upon the individual static and dynamic occlusion (Korioth et al. 1994a, b). Different types of loads also bring about different responses in bone. When erosive changes are found in the condyle, the trabecular bone volume (TBV) of the temporal portion of the joint is significantly higher (25%) than when the condyle is unchanged (16%; Flygare et al. 1997).
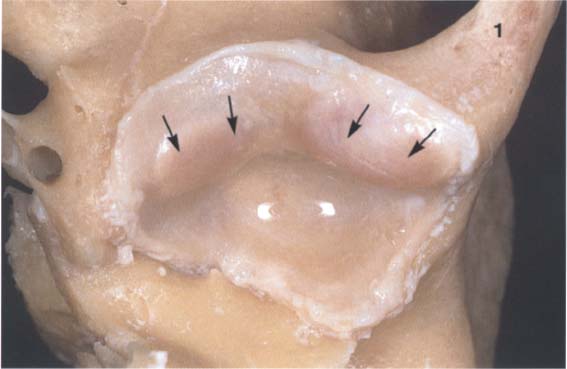
30 Inferior view of the temporal cartilaginous joint surface and capsule attachment
Caudal view of the left temporomandibular joint of a newborn. The bony portions have been separated from the periosteum up to the circular bilaminar zone. Part of the zygomatic arch (1) can be seen near the right border of the photograph. The fibrocartilaginous articular surfaces over the articular protuberance are thickened medially and laterally (arrows). When covered with synovial fluid they allow movements with virtually no friction (Smith 1982).
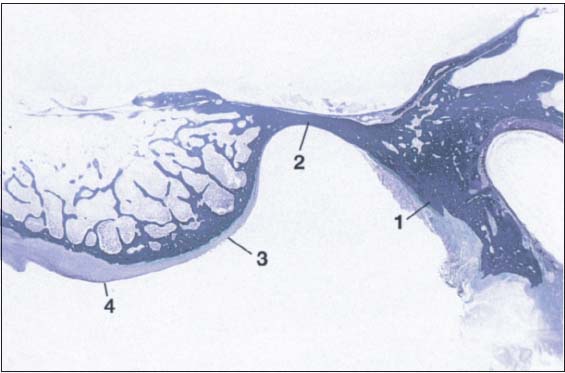
31 Sagittal histological section showing buildup of the temporal joint components
The temporal portion of the joint can be divided into four functional components: 1 postglenoidal process, 2 glenoid fossa, 3 articular protuberance, and 4 apex of the eminence. As a rule, no cartilage can be identified within the fossa. The average thickness of the fibrous cartilage over the protuberance and the eminence is between 0.07 and 0.5 mm (Hansson et al. 1977). As this photograph shows, there can be considerable variation in thickness within the same individual.
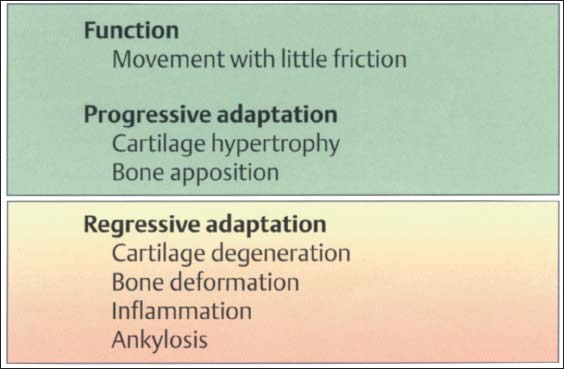
32 Function and structural adaptation of the articular eminence
A summary of the basic anatomical changes in the temporal joint tissues. Increased functional loading will cause hypertrophy through secondary cartilage formation and bone deposition (progressive adaptation). Persistent nonphysiological loading (massive influences) leads to deforming or degenerative changes. This regressive adaptation is accompanied by more or less noticeable rubbing sounds, some times in combination with pain.
Mandibular Condyle
Human condyles differ greatly in their shapes and dimensions (Solberg et al. 1985, Scapino 1997). From the time of birth to adulthood the medial-lateral dimension of the condyle increases by a factor of 2 to 2.5, while the dimension in the sagittal plane increases only slightly (Nickel et al. 1997). The condyle is markedly more convex in the sagittal plane than in the frontal plane.
The articulating surfaces of the joint are covered by a dense connective tissue that contains varying amounts of chondrocytes, proteoglycans, elastic fibers and oxytalan fibers (Hansson et al. 1977, Helmy et al. 1984, Dijkgraaf et al. 1995). The composition and geometric arrangement of the extracellular matrix proteins within the fibrous cartilage determine its properties (Mills et al. 1994 a, b). Cartilage that can absorb and distribute compressive loads is characterized by a matrix with high water content and high molecular weight chondroitin sulfate in a network of type II collagen (Maroudas 1972, Mow et al. 1992). A low level of functional demand upon the joint leads to an increase of type I collagen and a reduction of type II (Pirttiniemi et al. 1996). Interleukin la inhibits the matrix synthesis of chondrocytes, while the transforming growth factor TGF-b promotes it (Blumenfeld et al. 1997). The collagen fibers of the fibrocartilaginous joint surfaces are oriented mainly in a sagittal plane (Steinhardt 1934).
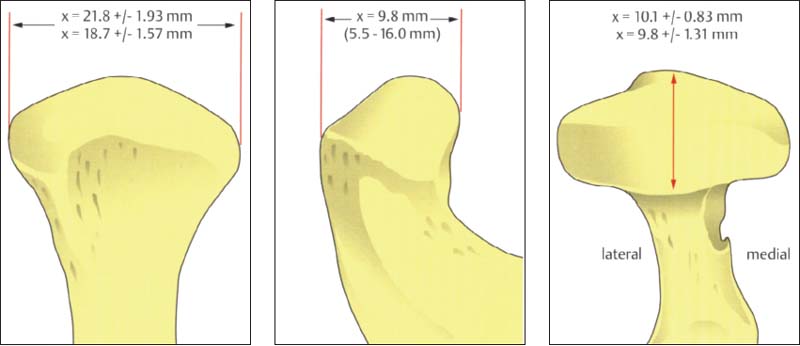
33 Condyle dimensions
Left: Width of condyle in the frontal plane (Solberg et al. 1985). The average condylar width is significantly greater in men (21.8 mm) than in women (18.7 mm).
Center: Anteroposterior dimension of the central portion shown in the sagittal plane (Öberg et al. 1971; minimum and maximum in parentheses).
Right: Anteroposterior dimension of the condyle in the horizontal plane. There is no significant difference between men (10.1 mm) and women (9.8 mm).
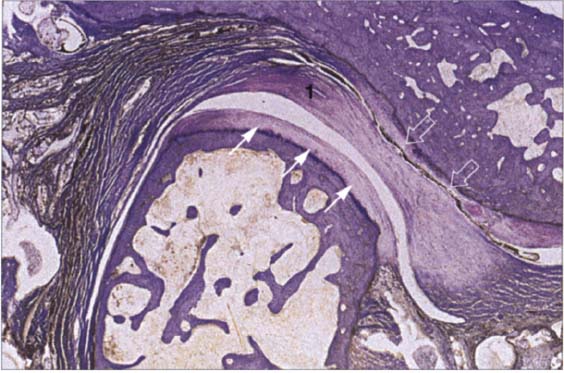
34 Functional joint surface
Histological preparation showing a physiological fibrocartilaginous joint surface (thin arrows) of the condyle of a 58-year-old individual. In spite of the intact joint surface on the condyle, the pars posterior (1) of the disk is flattened and the functional fibrocartilaginous temporal surface of the joint on the articular protuberance shows degenerative changes (outlined arrows). The subchondral cartilage has not yet been affected and would appear intact on a radiograph.
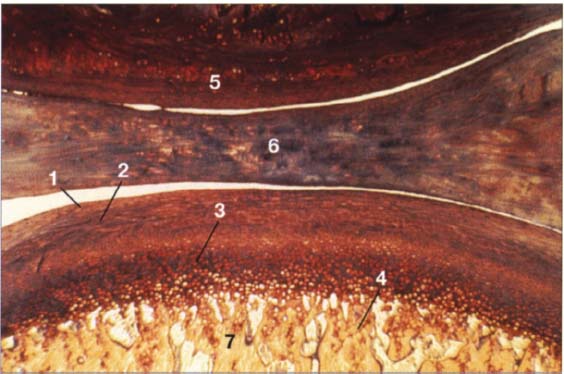
35 Buildup of the condylar cartilage
Histologically, the secondary cartilage of the condyle is made up of four layers:
1 Fibrous connective-tissue zone
2 Proliferation zone with undifferentiated connective-tissue cells
3 Fibrous cartilage zone
4 Enchondral ossification zone
Other structures shown are:
5 Eminence
6 Disk
7 Condyle
Contributed by R. Ewers
Joint surface cartilage must permit frictionless sliding of the articulating structures while at the same time it must be able to transmit compressive forces uniformly to the subchondral bone (Radin and Paul 1971). Hypomobility of the mandible results in a more concentrated loading of the joint surfaces. Even if the forces in the masticatory system remain the same, the load per unit of area on the cartilage will be increased when there is hypomobility. The amount of structural change depends upon the amplitude, frequency, duration, and direction of the loads (Karaharju-Suvanto et al. 1996).
In joints that have undergone erosive changes, the percentage of trabecular bone volume (21%) and the total bone volume (54%) are significantly higher than the corresponding 15% and 40% found in condyles without these changes (Flygare et al. 1997). Degenerative changes therefore are closely associated with nonphysiological loading of the joint surfaces.
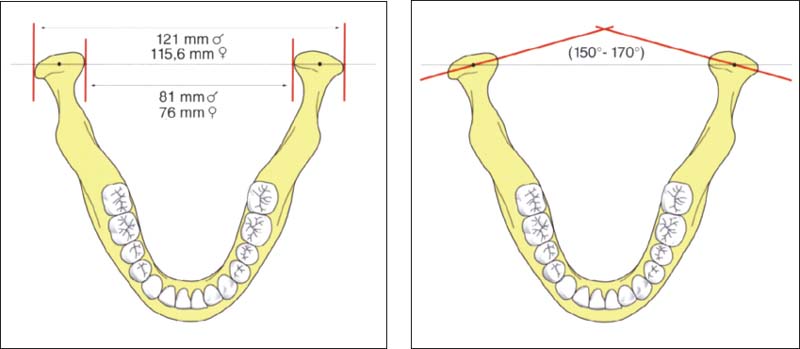
36 Intercondylar distance
Left: Sex-specific data on the distances between pairs of medial poles and lateral poles of the condyle (after Christiansen et al. 1987). The numbers given are average values. A difference of 5-10 mm in the Intercondylar distance will have a corresponding effect on the tracings of condylar movements and the accuracy of simulated movements in the articulator (see pp. 216 and 243).
Right: Schematic drawing illustrating the intercondylar angle.
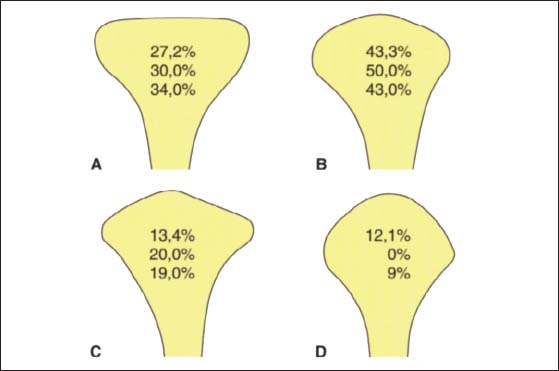
37 Condylar shapes in the frontal plane
According to Yale et al. (1963) 97.1% of all condyles fall into one of four groups based upon their frontal profile. These are described as either flat (A), convex (B), angled (C), or round (D). The relative frequencies of occurrence are taken from the works of Yale et al. (1963), Solberg et al. (1985), and Christiansen et al. (1987). The condyle form affects the radiographic image of this part of the joint in the Schüller projection (Bumann et al. 1999) and the loading of the joint surfaces (Nickel and McLachlan 1994).
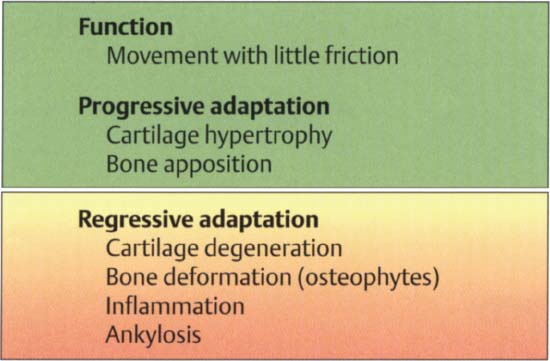
38 Function and structural adaptation of the condyle
Summary of the basic anatomical and functional changes in the condylar portion of the joint. Increased functional loading will stimulate cartilaginous hypertrophy (= progressive adaptation) that is not noticeable clinically. Continuous nonphysiological loading of the condyle can lead to degeneration, deformation, and even ankylosis (Dibbets 1977, Stegenga 1991). These changes may be accompanied by pain or, with sufficient adaptation, they may progress painlessly.
Positional Relationships of the Bony Structures
The position of the condyle relative to the articular protuberance has been a subject of controversy in dentistry for many years (Lindblom 1936, Pullinger et at. 1985). A well-defined condylar position oriented to the maximal occlusion is especially relevant to extensive dental treatment (Spear 1997). In the past, to transfer the jaw relations to an articulator the condyles were always placed in their most posterosuperioi position because this relationship could be most easily reproduced (Celenza and Nasedkin 1979). Under purely static conditions the condylar position is dependent upon the shape of the fossa, the inclination of the protuberance, and the shape of the condyle. In the 1970s this led to the assignment of a geometric centric position of the condyle in the fossa (Gerber 1971). However, the dimensions of the joint space are quite variable in both the sagittal plane (anterior, posterior, and superior) and the transverse plane (medial, central, and lateral) (Pullinger et al. 1985, Hatcher et al. 1986, Christiansen et al. 1987, Bumann et al. 1997). For this reason the concept of an anatomical orientation is untenable, and the radiographic techniques (p. 148) are unsuitable for determining a therapeutic condylar position (Pullinger and Hollender 1985). Therefore the current definitions of centric relation are geared more toward the functional conditions (van Blarcom 1994, Dawson 1995, Lotzmann 1999). It has been demonstrated experimentally that the surfaces of the temporomandibular joint are subjected to loads of 5-20 N (Hylanderl979, Brehnan et al. 1981, Christensen et al. 1986). In a patient’s habitual occlusion this force is partially intercepted by the occluding premolars and molars. Tooth loss can lead to higher joint loading and regressive adaptation (van den Hemel 1983, Christensen et al. 1986, Seligman and Pullinger 1991). However, if the joint’s capacity for adaptation is sufficiently great, degenerative changes may be avoided (Helkimo 1976. Kirveskari and Alanen 1985. Roberts et al. 1987). The direction of functional loading is anterosuperior against the articular protuberance (Dauber 1987). Clear evidence for this is the presence of the load-induced secondary cartilage on the joint surfaces in this region.
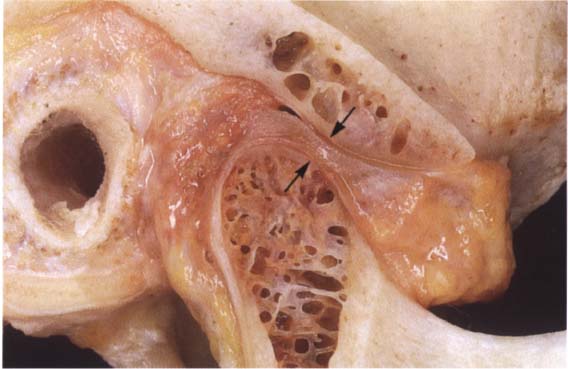
39 Sagittal relationships
Macroscopic anatomical preparation showing the relation of the fossa, disk, and condyle to one another in the sagittal plane. Because the shapes of fossae and condyles vary so greatly, it is not possible to determine a universally applicable measurement of the condylar position. Although the physiological (i.e. centric) condylar position is defined as the most anterosuperior position with no lateral displacements (arrows), this position depends upon the basic neuromuscular tonus.
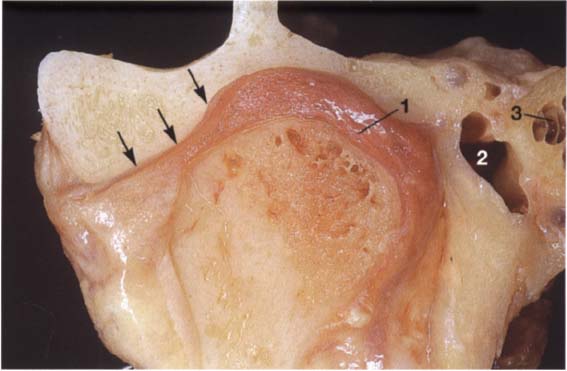
40 Frontal relationships
Macroscopic anatomical preparation showing the relation of the fossa, disk, and condyle to one another in the frontal plane. In this plane, too, there is no standard geometric arrangement of condyle and fossa because of the variability of the hard and soft tissues (Yung et al. 1990). In this preparation the disk (arrows) is displaced laterally. Structures of the bilaminar zone (1) can be identified in the medial portion of the joint. The close proximity of the joint to the middle (2) and inner ear (3) can also be observed.
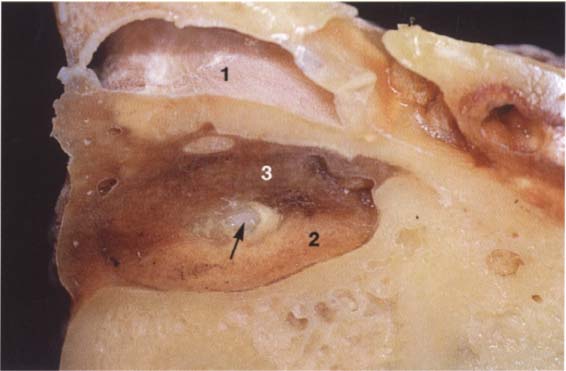
41 Horizontal relationships
A right temporomandibular joint viewed from above showing the relation of the fossa, disk, and condyle to one another in the horizontal plane. The lateral portion of the joint is near the left border of the picture. Near the upper border a section through the external auditory meatus can be seen (1). The roof of the fossa has been removed. Near the center of the picture lies the transition from the pars posterior (2) to the bilaminar zone (3). The central perforation was created during sectioning, and through it can be seen the upper surface of the condyle (arrow).
Positioning of the condyles on the protuberances is accomplished exclusively through the antagonistic activity of the neuromuscular system and from a functional standpoint requires no border position.
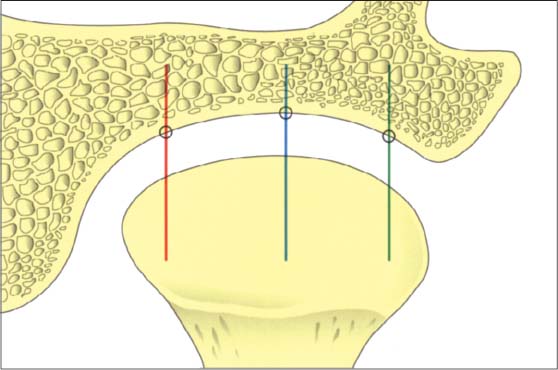
42 Relationships in the frontal plane
Schematic depiction of the joint space relationships in the frontal plane. A number of studies have reported that the dimensions found in the lateral, central, and medial parts may vary greatly (Christiansen et al. 1987, Vargas 1997). Although the lateral portion is affected more frequently by degenerative changes, the width of the joint space is usually least at its center (blue line).
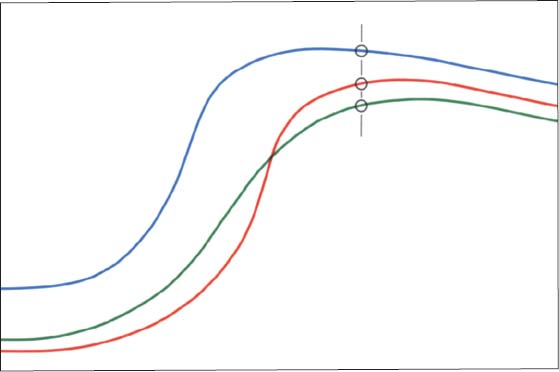
43 Contours on the temporal surface of the joint
Schematic drawing (modified from Hasso et al. 1989) of the contours in the lateral (green), central (blue), and medial (red) regions of the joint. The entire protrusive functional path is represented as a convex bulge that can vary markedly as the result of regressive or progressive adaptation. Therefore, the loads borne by the lateral and medial portions of the joint during function are also influenced by the morphology of the articular protuberance (Öberg et al. 1971, Hylander 1979. Hinton 1981).
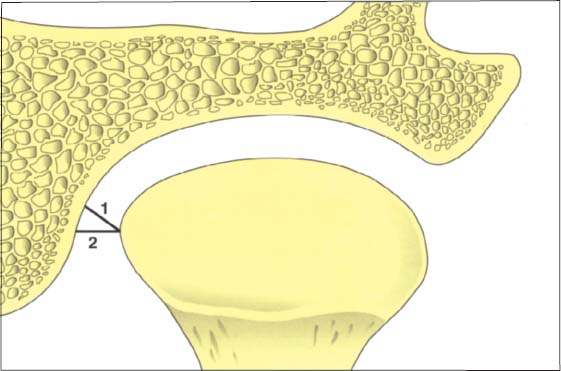
44 Relationships in the medial part of the joint
Schematic drawing (modified from Christiansen et al. 1987) of the positional relationships in the medial portion of a left temporomandibular joint. This finding also emphasizes the fundamental principles of physiological joint movements. As with all other joints, the temporomandibular joint has a passive “play” space in all directions and is thus not confined to any border position. Average values: 1 = 3.4 mm; 2 = 4.4 mm
Articular Disk
The articular disk can be divided into three regions based upon their function: the partes anterior, intermedia, and posterior. The primary functions of the disk are to reduce sliding friction and to dampen load spikes (McDonald 1989, Scapino et al. 1996). The extracellular matrix of the Fibrocartilaginous disk consists primarily of type I and type II collagen (Mills et al. 1994b). The orientation of the collagen fibers in the disk displays a typical pattern (Knox 1967, Scapino 1983). In the pars intermedia dense bundles of collagen fibers run approximately in a sagittal direction. These intertwine with the exclusively transverse fibers of the pars anterior and pars posterior (Takisawa et al. 1982). Elastic fibers are found in all parts of the disk (Nagy and Daniel 1991) but are more numerous in the pars anterior and in the medial portion of the joint (Luder and Babst 1991). A reduction in the thickness of the disk results in an exponential increase in the load it experiences (Nickel and McLachlan 1994). The more rapidly a load is applied, the “stiffer” the disk reacts (Chin et al. 1996). The inferior stratum and the convexity of the pars posterior help stabilize the disk on the condyle.
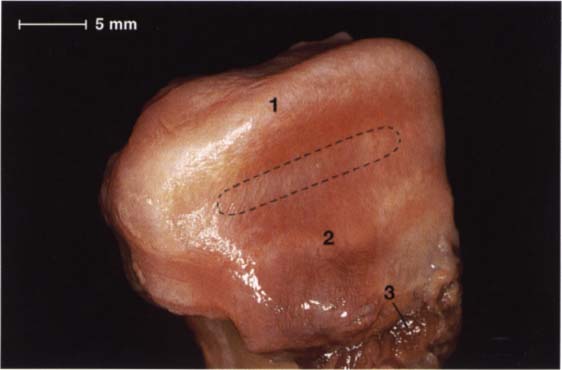
45 Alignment of fibers within the disk and their attachment to the condyle
Macroscopic anatomical preparation of the disk-condyle complex of a right temporomandibular joint The collagen fibers of the pars posterior (1) and the pars anterior (2) run from the medial to the lateral pole of the condyle (Moffet 1984), making possible a wide range of movement of the disk relative to the condyle in the sagittal plane. The fibers of the pars intermedia (outlined area), on the other hand, run in a more sagittal direction. The medial pterygoid muscle (3) makes its insertion at the anteromedial region.
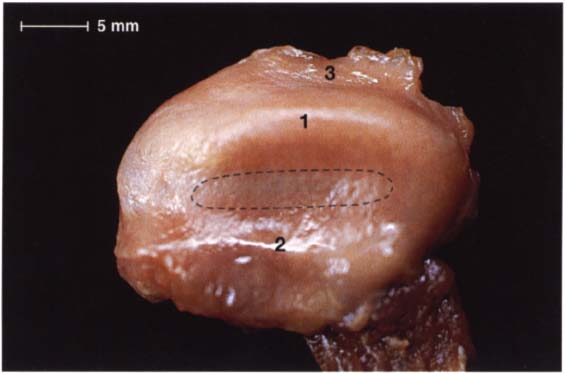
46 Cranial view
A view from above of the disk in Figure 45 after removal of the condyle, the fibers in the pars posterior (1) and pars anterior (2) can be seen more clearly. Histologically the disk is composed of dense collagenous connective tissue with a few embedded chondrocytes (Rees 1954). In the pars anterior and pars posterior the chondrocytes are found in clusters, but in the pars intermedia (outlined) they are arranged uniformly. Part of the bilaminar zone (3) can be seen attached at the distal border of the pars posterior.
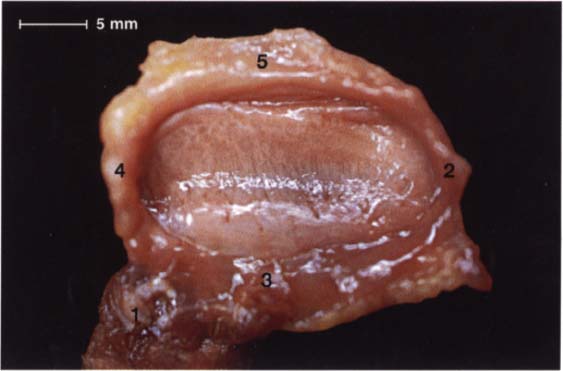
47 Inferior view of the same disk
In this view the insertion of a portion of the superior head of the lateral pterygoid muscle (1) can be clearly seen. The remaining fibers of the superior head insert on the condyle. This preparation also demonstrates the insertion of the lateral (2), anterior (3), and medial (4) borders of the joint capsule. In the posterior part of the joint the capsule is connected to the posterior surface of the condyle by the stratum inferium (5) of the bilaminar zone (see p. 47).
Anatomical Disk Position
In a physiological temporomandibular joint, the pars posterior of the disk lies on the superior portion of the condyle. In the “centric condylar position” the thinnest part of the disk, the pars intermedia, is located between the anterosuperior convexity of the condyle and the articular protuberance (van Blarcom 1994). This finding is also supported by studies using measurements and mathematical models (Bumann et al. 1997, Kubein-Meesenburg 1985). The pars anterior lies in front of the condyle (Steinhardt 1934, Wright and Moffet 1974, Scapino 1983). The disk is attached to the medial and lateral poles of the condyle by means of the transversely aligned collagen fibers of the pars anterior and pars posterior. Viewed by itself, this anatomical arrangement with the condyle allows a great degree of movement during active mandibular movements (see p. 46). The disk exhibits viscoelastic properties under compressive loads. Its resistance is strengthened by the arrangement of the collagen fibers (Shengyi and Xu 1991). The elastic fibers within the disk serve primarily to restore the shape of the disk after a load has been removed (Christensen 1975).
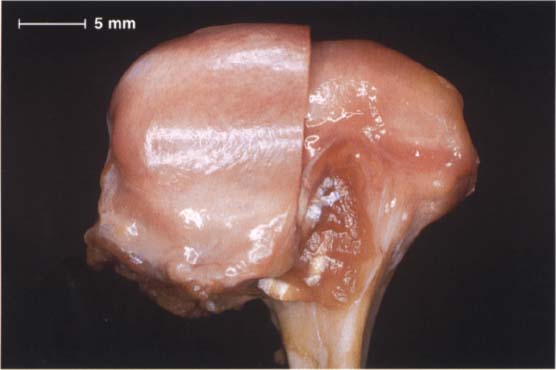
48 Anterosuperior aspect of the disk-condyle complex
Macroscopic anatomical preparation of a left temporomandibular joint showing the relationship between disk and condyle. The lateral half of the disk has been removed for a clearer view. The dorsal border of the pars posterior is near the region of the apex of the condyle. From a functional point of view, this broad description is not very helpful for diagnostic purposes because the physiological position of the pars posterior depends to a large extent upon the inclination of the protuberance.
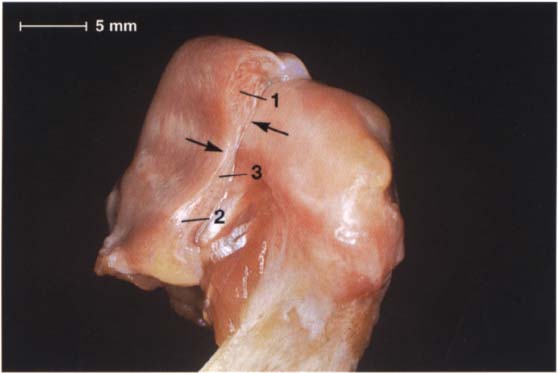
49 Anterolateral aspect of the disk-condyle complex
The same preparation in half profile. Here the pars posterior (1), pars intermedia (3), and pars anterior (2) can be clearly distinguished. Although the posterior border of the pars posterior lies over the apex of the condyle, the pars intermedia is in front of the anterosuperior convexity (arrows) of the condyle. The pars anterior is 2.0 mm thick, the pars intermedia 1.0 mm thick, and the pars posterior 2.7 mm thick (Gaa 1988).
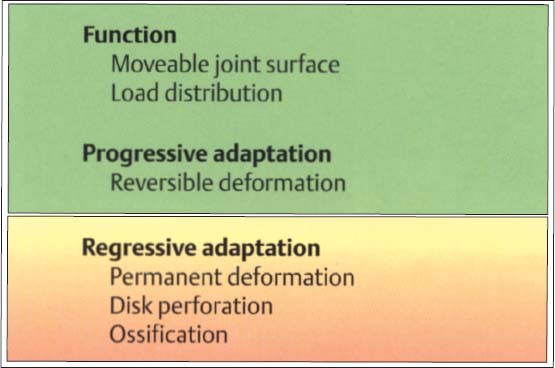
50 Function and structural adaptation of the disk
Functionally, the disk serves as a “moveable fossa” for the condyle. Because of its unique tissue structure it can cushion and dampen peaks of force. Progressive adaptation differs from regressive in that the former is reversible. Strictly speaking, there is no “positive” tissue reaction in the disk because functional loads as well as continuous nonphysiological loads result in deformation.
Bilaminar Zone
The posterior portion of the temporomandibular joint has been variously referred to as the bilaminar zone (Rees 1954), retroarticular plastic pad (Zenker 1956), retroarticular pad (DuBrul 1988), retrodiskal fat pad (Murikami and Hoshino 1982), or trilaminar zone (Smeele 1988). It consists of an upper layer (superior stratum) and a lower layer (inferior stratum) (Rees 1954. Griffin and Sharpe 1962). Between these two layers lies the genu vasculosum with its numerous vessels, nerves, and fat cells (Griffin and Sharpe 1962). The superior stratum is composed of a loose network of elastic and collagen fibers, fat, and blood vessels (Zenker 1956). By contrast, the inferior stratum is made up of tight collagen fibers (Rees 1954, Wilkes 1978, Luder and Bobst 1991). In the bilaminar zone the collagen fibers are more loosely organized and run more or less in the sagittal plane (Mills et al. 1994 b). The fibers of both strata stream into the pars posterior of the disk and there intertwine with the transverse fibers of the pars posterior and the sagittal fibers of the pars intermedia (Scapino 1983). The elastic fibers in the bilaminar zone have larger diameters than those of the disk and are concentrated predominantly in the superior stratum (Rees 1954, Scapino 1983, Mills et al. 1994a).
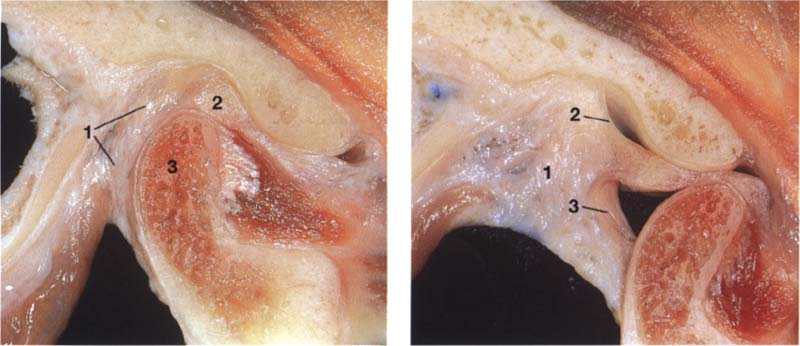
51 Macroscopic anatomical preparation
Left: With the jaws closed the bilaminar zone (1) fills the space posterior to both the pars posterior (2) I and the condyle (3). The inferior stratum stabilizes the disk on the condyle in the sagittal plane. An overextension of the bilaminar zone through posterosuperior displacement of the condyle is an essential precondition for an anterior disk displacement to occur.
Right: With the mouth open the genu vasculosum (1) fills with blood. The superior stratum (2) and inferior stratum (3) can be easily identified.
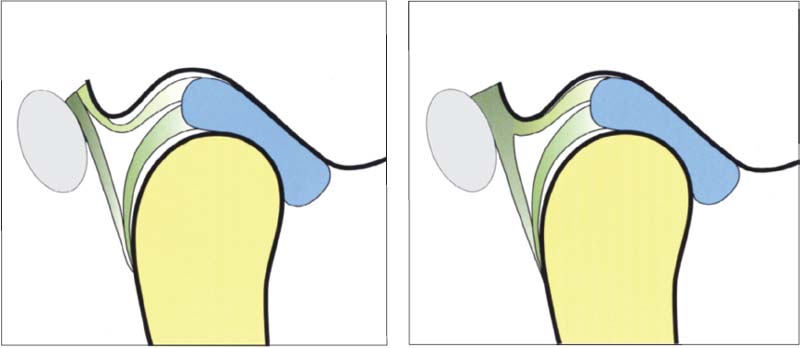
52 Variants of the postero-superior attachment
Left: Type A insertion. The superior stratum and the posterior joint capsule run separately to their insertions in the fissures. This type of insertion occurs most often in the medial portio/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


