The use of implantable biomaterials and devices plays a critical role in reconstruction of most traumatic facial injuries, particularly those of the underlying bony skeleton. Significant advances in materials science and engineering during the latter half of the 20th century have made the internal use of alloplastic implants an integral part of many primary and secondary facial procedures. Their use can also be traced to the simultaneous development of broad-spectrum antibiotics, an improved understanding of the healing of bone and soft tissues, and the remarkable tolerance of well-vascularized facial tissues to alloplastic materials.
Alloplastic implants are made from a wide array of biomaterials and have diverse physical structures and properties, principally dictated by their role, broadly those used for short term use to aid healing, or long term deformity. The facial surgeon may have difficulty interpreting the merits of the particular biomaterial and its appropriateness for the specific facial site. In the future, novel biomaterials will be fabricated, and pharmacological technology (e.g., antibiotics, growth factors) will be merged with existing biomaterials to produce new types of surgical implants. Selection of a synthetic implant should be based on knowledge of its chemical composition, its physical structure, and the proposed site of tissue implantation. Surgeons must also look critically at manufacturer’s claims, recognizing that the implant will be in place for many years.
Alloplastic Materials, Biocompatibility, and Wound Healing
Alloplastic materials can be described by the term synthetic , indicating that they are manufactured from nonorganic sources. They should not be confused with allografts, heterografts, or xenogeneic materials, which are derived from organic sources and represent a completely different type of surgical implant that carries different risks from those of alloplastic materials (e.g., immunological rejection, transmission of viral diseases). Alloplastic implants provide an array of reconstructive materials that offer solutions to many facial needs, and their use often simplifies the operative procedure in terms of time and complexity of technique.
For an alloplastic material to be clinically successful, it must be biocompatible, entailing an acceptable interaction between the host and the implanted material. The level of material biocompatibility is influenced by several major factors, including the host reaction to the physical characteristics of the implant material, the tissue site of implantation, and the surgical technique of placement. The difficulty in developing consistent and long-term biomaterial success after implantation underscores the complex interactions between an implant and the body and explains why so few safe and effective biomaterials exist despite the tremendous advances that have been made in biomaterial development and engineering during the past 50 years.
The end-stage healing response to most biomaterials is the formation of an enveloping fibroconnective tissue scar or fibrous encapsulation. This reaction is initiated with the surgical implantation procedure, which generates an acute inflammatory response due to the induced tissue damage; this is followed by a cascade of events, including chronic inflammation, granulation tissue development, foreign body reaction, and ultimately an enveloping fibrosis. The fibrous capsule represents the body’s reparative response, which is to separate the body from the foreign material, and is essentially a biologic barrier between self and nonself. Almost all biomaterials implanted in the face develop a surrounding fibrous scar, with the one exception of metallic plates used for bone fixation, which can develop bone attachment directly to the implant.
Principles of Facial Alloplastic Material Selection and Surgical Placement
Although the composition of implanted alloplastic material has an impact on biocompatibility, the anatomic location of placement and the surgical technique used to place the implant have an equal or greater effect on long-term clinical success. Ensuring that the biomaterial is appropriately matched to the tissue plane within which it will be implanted is ultimately the responsibility of the surgeon.
When the tissue quality of the recipient site is initially assessed, emphasis is placed on vascularity and adequacy of soft tissue coverage. Decreased vascularity due to scar or prior operations or irradiation compromises the establishment of a normal fibrovascular tissue encapsulation and significantly limits a proper inflammatory response if the surface of the biomaterial becomes inoculated or infected secondarily. Soft tissue coverage over an implant should be as thick as possible, because the thinner the overlying tissue coverage, the greater the likelihood over time that implant exposure or extrusion may occur. Alloplastic implants that are more deeply placed (e.g., subperiosteal, submuscular plane) rarely develop exposure. Implants placed immediately under the skin or with thin overlying subcutaneous fat eventually may develop thinning of the skin, particularly if the material lacks sufficient flexibility or if it is placed in an area of significant tissue mobility. In either case, the overlying dermis of the skin thins due to pressure of the underlying avascular implant. Placement of an implant into or through a tissue plane of existing or recent contamination significantly increases the risk of subsequent infection. Because most alloplastic implants never establish an intramaterial vascular supply and have an affinity for bacterial adhesion, alloplastic tolerance is very low for wounds that are contaminated or are in contact with facial sinuses. Fortunately, implant placement in the face is fairly forgiving.
The size of the implant should be considered in relation to that of the tissue pocket or wound cavity. An implant that places the surrounding soft tissue under tension is more likely to extrude or become exposed, particularly if there are other adverse tissue or implant characteristics. In certain clinical situations, the overlying soft tissue can safely stretch and expand to accommodate large biomaterial placements. However, this is most safely done when the implant has a thick overlying soft tissue layer or is placed in the submuscular plane.
Implant mobility should be minimized by fixation to the most stable adjacent structure whenever practical or be placed in a well-contained, healthy tissue pocket. This ensures the desired postoperative implant position and prevents migration or exposure of the implant to other, less desirable tissue planes.
Patients undergoing alloplastic facial implantation should receive an intravenous antibiotic infusion during placement, followed by an oral course postoperatively. Other than coverage for Staphylococcus or Streptococcus , depending on the path of insertion (e.g., intraoral, transcutaneous, transconjunctival), no specific antibiotic or duration of administration has been shown to have a superior clinical advantage. The rationale for antibiotic coverage is to prevent or eliminate any bacterial inoculation that may have occurred on the implant surface. No large clinical trials have been conducted to confirm that this is true, but it appears to have no compelling disadvantage. Additional antibiotic coverage for certain types of facial implants is often sought by washing or soaking of the implant before intraoperative insertion. The value of this technique is best determined by the hydrophilicity or wetting ability of the implant material. Increased hydrophilic capacity of a biomaterial allows more antibiotic solution to be drawn into the implant. Whether this antibiotic impregnation lowers the postoperative infection rate is unknown, but this intraoperative technique is widely used, particularly for nonmetal implants. With less hydrophilic or overtly hydrophobic biomaterials, antibiotic soaking only mechanically removes any bacteria or contamination that has inadvertently become attached to the implant surface during the placement process, and it is likely to be no more effective than washing with any nonantibiotic solution.
Intraoperative handling of the implant is associated with the risk of postoperative infection. Extensive handling or exposure of the implant before insertion should be avoided. The implant should not be removed from its sterile packaging until the pocket or recipient site has been fully dissected and irrigated. Once removed from its sterile package or container, the implant should be handled by instruments and have minimal contact with the contaminated gloved hand. Ideally, new gloves should be used if the implant is to be manually handled. Implant contact with the surrounding skin or oral cavity should be minimized to prevent a final source of bacterial transmission onto the implant surface. Whether these intraoperative techniques decrease the risk of postoperative infection is difficult to prove, but they are reasonable and prudent precautions to decrease potential postoperative complications.
Principles of Facial Alloplastic Material Selection and Surgical Placement
Although the composition of implanted alloplastic material has an impact on biocompatibility, the anatomic location of placement and the surgical technique used to place the implant have an equal or greater effect on long-term clinical success. Ensuring that the biomaterial is appropriately matched to the tissue plane within which it will be implanted is ultimately the responsibility of the surgeon.
When the tissue quality of the recipient site is initially assessed, emphasis is placed on vascularity and adequacy of soft tissue coverage. Decreased vascularity due to scar or prior operations or irradiation compromises the establishment of a normal fibrovascular tissue encapsulation and significantly limits a proper inflammatory response if the surface of the biomaterial becomes inoculated or infected secondarily. Soft tissue coverage over an implant should be as thick as possible, because the thinner the overlying tissue coverage, the greater the likelihood over time that implant exposure or extrusion may occur. Alloplastic implants that are more deeply placed (e.g., subperiosteal, submuscular plane) rarely develop exposure. Implants placed immediately under the skin or with thin overlying subcutaneous fat eventually may develop thinning of the skin, particularly if the material lacks sufficient flexibility or if it is placed in an area of significant tissue mobility. In either case, the overlying dermis of the skin thins due to pressure of the underlying avascular implant. Placement of an implant into or through a tissue plane of existing or recent contamination significantly increases the risk of subsequent infection. Because most alloplastic implants never establish an intramaterial vascular supply and have an affinity for bacterial adhesion, alloplastic tolerance is very low for wounds that are contaminated or are in contact with facial sinuses. Fortunately, implant placement in the face is fairly forgiving.
The size of the implant should be considered in relation to that of the tissue pocket or wound cavity. An implant that places the surrounding soft tissue under tension is more likely to extrude or become exposed, particularly if there are other adverse tissue or implant characteristics. In certain clinical situations, the overlying soft tissue can safely stretch and expand to accommodate large biomaterial placements. However, this is most safely done when the implant has a thick overlying soft tissue layer or is placed in the submuscular plane.
Implant mobility should be minimized by fixation to the most stable adjacent structure whenever practical or be placed in a well-contained, healthy tissue pocket. This ensures the desired postoperative implant position and prevents migration or exposure of the implant to other, less desirable tissue planes.
Patients undergoing alloplastic facial implantation should receive an intravenous antibiotic infusion during placement, followed by an oral course postoperatively. Other than coverage for Staphylococcus or Streptococcus , depending on the path of insertion (e.g., intraoral, transcutaneous, transconjunctival), no specific antibiotic or duration of administration has been shown to have a superior clinical advantage. The rationale for antibiotic coverage is to prevent or eliminate any bacterial inoculation that may have occurred on the implant surface. No large clinical trials have been conducted to confirm that this is true, but it appears to have no compelling disadvantage. Additional antibiotic coverage for certain types of facial implants is often sought by washing or soaking of the implant before intraoperative insertion. The value of this technique is best determined by the hydrophilicity or wetting ability of the implant material. Increased hydrophilic capacity of a biomaterial allows more antibiotic solution to be drawn into the implant. Whether this antibiotic impregnation lowers the postoperative infection rate is unknown, but this intraoperative technique is widely used, particularly for nonmetal implants. With less hydrophilic or overtly hydrophobic biomaterials, antibiotic soaking only mechanically removes any bacteria or contamination that has inadvertently become attached to the implant surface during the placement process, and it is likely to be no more effective than washing with any nonantibiotic solution.
Intraoperative handling of the implant is associated with the risk of postoperative infection. Extensive handling or exposure of the implant before insertion should be avoided. The implant should not be removed from its sterile packaging until the pocket or recipient site has been fully dissected and irrigated. Once removed from its sterile package or container, the implant should be handled by instruments and have minimal contact with the contaminated gloved hand. Ideally, new gloves should be used if the implant is to be manually handled. Implant contact with the surrounding skin or oral cavity should be minimized to prevent a final source of bacterial transmission onto the implant surface. Whether these intraoperative techniques decrease the risk of postoperative infection is difficult to prove, but they are reasonable and prudent precautions to decrease potential postoperative complications.
Alloplastic Implant Types
Although many types of implants have been used over the past 25 years, only some classifications of biomaterials have a significant clinical history of successful use for soft or hard tissue replacement and repair. Several biomaterials are commercially available for surgical implantation: dimethylsiloxane, polytetrafluoroethylene, polyethylene, polyester, polyamide, and acrylic polymers; titanium and gold metals; calcium phosphate–based biomaterials; and cyanoacrylate adhesives.
Silicone
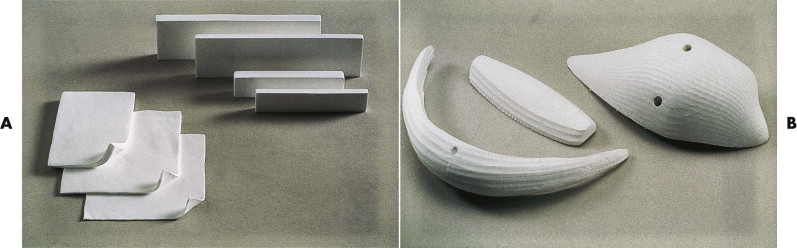
The use of dimethylsiloxane (silicone) is widespread throughout many areas of medicine and surgery and is associated with a remarkable paucity of significant adverse tissue reactivity. It is used in the face primarily as onlay implants for reconstruction of zygomatic, maxillary, nasal, and mandibular contours ( Fig. 8-1 ).

Silicone is a polymer created from interlinking of silicon and oxygen (positions 14 and 8, respectively, on the periodic table of chemical elements) with methyl side groups; it is the only noncarbon chain polymer used in medical implantation devices. The backbone of this polymer has alternating monomers of dimethylsiloxane, SiO(CH 3 ) 2 , and is extremely resistant to degradation in the body due to the very strong and stable silicon-oxygen bonds. When the monomers of dimethylsiloxane are linked together, polydimethylsiloxane is formed, and the amount of crosslinking between different strands of polydimethylsiloxane results in various physical forms. Minimal crosslinking produces a gel, which was commonly used in the past as the filler material for breast implants. When combined with silica particles and other chemical reagents, a silicone gel can be converted (i.e., vulcanized) into a solid rubber. The varying elasticity of silicone rubber gives it great clinical versatility for use as various facial implants. The excellent biocompatibility of silicone materials in the body may have some relation to its proximity to carbon (position number 6) on the periodic table of chemical elements.
Implants composed of solid silicone represent one of the earliest alloplastic materials used with extensive applications for facial skeletal augmentation procedures. Although the material was initially developed for use in the chin, an extensive array of implants have become available for every conceivable facial site, including the parasymphysis; inferior border and mandibular ramus (angle); paranasal, infraorbital, maxillary, and malar sites; orbital floor and globe; nasal dorsum and columella; and ear (see Fig. 8-1 ). Solid silicone offers several advantages: it is easy to sterilize by steam autoclaving or irradiation without degradation of the implant; it is easily modified intraoperatively by scalpel or scissors; it retains its flexibility through a wide temperature range; it can be stabilized by suture or screw fixation through the implant; and it is economical.
Solid silicone has a high degree of chemical inertness, is hydrophobic, and is extremely resistant to degradation. No significant clinical toxicity or allergic reactions appear to exist. Tissue ingrowth or attachment to the implant does not occur, and it acts as a relatively inert filler with a predictable surrounding fibrous encapsulation that may change very little or not at all over a long period of implantation. When the implant is exposed to mechanical loading, fragmentation of the material and a synovitis may occur because of its poor mechanical properties. Therefore, it should not be used in the temporomandibular joint as an arthroplastic or interface material.
Polytetrafluoroethylene
The perfluorocarbons represent a very biocompatible group of carbon-based biomaterials that are used in almost every specialty of surgery and in dentistry. They have an ethylene (carbon) backbone to which is attached four fluorine molecules, producing polytetrafluoroethylene (PTFE).
The bonding of highly reactive fluorine to carbon creates an extremely stable biomaterial that is not biodegradable in the body due to the lack of any known human enzyme that can disrupt the fluorine-carbon bonds. In addition to its chemical stability, its surface is very nonadherent, with significant antifrictional properties. Because of the lack of crosslinking in its molecular structure, it is very flexible and has a low tensile strength.
PTFE was originally introduced in facial surgery in the 1980s as a skeletal augmentation material known as Proplast; it was combined with graphite (Proplast I), alumina (Proplast II), or hydroxyapatite (Proplast-HA) as preformed or block facial implants. It is no longer available in the United States. It was withdrawn after a misconceived approach of using it in the temporomandibular joint as a meniscal replacement or as part of a glenoid fossa or condylar joint prosthesis, where it was exposed to mechanical loads resulting in delamination, material fragmentation, particulation, and subsequent foreign body reactions.
However, PTFE has been reborn as a subcutaneous augmentation material (SAM) (W.L. Gore and Associates, Flagstaff, Ariz). Based on the manufacturer’s extensive experience with other surgical implants composed of PTFE (e.g., vascular prostheses, soft tissue patches, sutures), a variety of blocks, preformed implants, strips, and strands are available for facial augmentation from subperiosteal to subdermal placement ( Fig. 8-2 ). The material is composed of fine, expanded PTFE fibrils that are oriented and held together by solid pieces of the same material. The fibrillar composition results in non-interconnected surface openings with pore sizes of 10 to 30 µm. This allows for some soft tissue ingrowth, less fibrous encapsulation, and little tendency for migration. The material is easily shaped with scalpel and scissors, may be resterilized (stable at temperatures up to 325° F) if not used, threads easily through subcutaneous tissue and into tissue pockets, and can be anchored to adjacent tissues by sutures or screws.
With its long history of use as a vascular prosthesis since 1975 and in other abdominal and thoracic surgery applications, its clinical safety is well established, and extensive histologic evaluations of its tissue response have been done. It has been approved as an implant material for facial applications since 1994 and has been widely employed for subdermal implantation in the lip, nasolabial folds, glabella, nasal dorsum, and other subcutaneous facial defects; as slings for ptotic tissues of the eyelid and face; and for bony augmentation of the midface, malar, and mandibular areas. In block form, its compressive deformability by handling has been improved by the addition of reinforcement layers. Its ease of removal in subcutaneous sites due to the lack of significant ingrowth offers an advantage in the event of infection or if additional augmentation or modification of the material is required secondarily. In areas of thin skin with little subcutaneous substance (e.g., nasal dorsum), PTFE, like all other inorganic materials, should be used cautiously because of the higher potential for complications.
Polyethylene
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


