9
Biomaterials
INTRODUCTION
Definitions and terminology
In dentistry, most clinical wear events are categorized on the basis of visual inferences of the principal causes of the events. As such, we end up with the main categories introduced in Chapter 1 of attrition, erosion, and abrasion. At the microscopic level, many more things may actually be happening than can be described by these simple terms. In materials science and engineering, wear is classified on the basis of microscopic mechanisms occurring at the surface of interest. While there are strong similarities between the dental and engineering classifications, there are also some exceptions that need to be recognized (Fig. 9.1 & Table 9.1). Attrition is associated predominantly with physical properties of a material (e.g. friction and adhesion). Erosion, in materials engineering parlance, is associated mainly with a collection of chemical properties that include solubility/disintegration, chemical corrosion, electrochemical corrosion, and etching, but do not always involve acids. Abrasion is heavily aligned with the mechanical response of a substrate in response to an opponent solid or a solid particle.
Figure 9.1 Superposition of dental and engineering wear events to include material properties likely to be involved, intraoral wear situations, and environmental factors influencing wear rates.
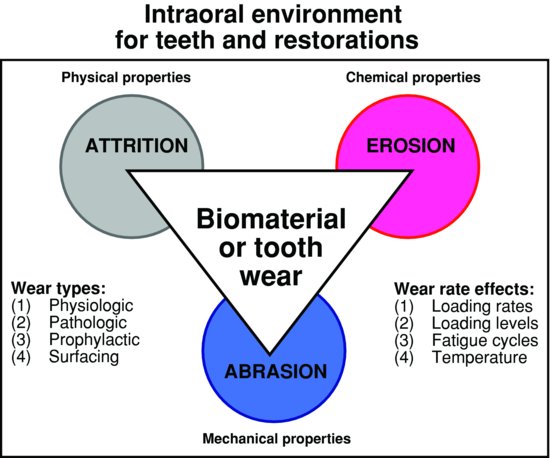
Table 9.1 Comparison of wear definitions from dentistry and engineering.a
| Dentistry (Chapter 1) | Engineering (quoted in part from ASM 1992, pp. 1–20) |
| Wear: Loss of tooth substance or restorative material as a function of normal or abnormal intraoral events | Wear: Damage to a solid surface, generally involving progressive loss of materials, which is due to relative motions between that surface and a contacting substance or substances |
| Attrition: Attrition involves two-surface wear, tooth to tooth related | Attrition: Removal of small fragments of surface material during a sliding contact |
| Erosion: Erosion, less commonly referred to as corrosion, results from acidic dissolution of mineralised tooth structure | Erosion (erosive wear): (1) Loss of material from a solid surface due to the relative motion in contact with a fluid that contains solid particles (abrasive erosion = parallel; impingement erosion or impact erosion). (2) Progressive loss of original material from a solid surface due to mechanical interaction between that surface and a fluid, a multicomponent fluid, and impinging liquid or solid particles Erosion–corrosion: A conjoint action involving corrosion and erosion in the presence of a corrosive substance |
| Abrasion: Abrasion on a surface comprises wear from externally applied particles or objects | Abrasion: A process in which hard particles or protuberances are forced against and moving along a solid surface. Note: Sometimes this term is used to refer to abrasive wear. See also ‘abrasive erosion’ Abrasive erosion: Erosive wear caused by the relative motion of solid particles which are entrained in a fluid, moving nearly parallel to the solid surface. See also ‘erosion’ Abrasive wear: Wear by the displacement of materials caused by hard particles or hard protuberances. Wear due to hard particles or hard protuberances forced against and moving along a solid surface |
| aNote that dental erosion presumes that acid is present and the engineering definition just assumes that liquid is present. | |
Other areas of this text emphasize that all intraoral events are multifactorial processes. A method to consider all of these combinations is to plot the degree of involvement of each corner process (see the triangle in the middle of Fig. 9.1). Any event that is primarily erosion would be plotted inside the triangular zone closest to the erosion corner. Something representing an equal combination of erosion and attrition would be plotted half-way along the line connecting those two corners. Something deemed an equal combination of all three processes would be plotted exactly in the middle of the triangle.
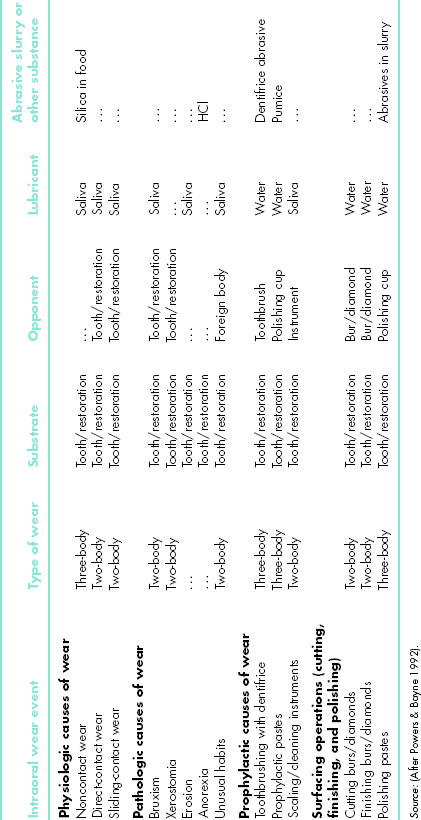
Wear events also may be grouped as physiologic, pathologic, prophylactic, and surfacing ones (Table 9.2). Physiologic are ones associated with intraoral functions. Pathologic ones result from disease or dysfunctional patterns. Prophylactic ones occur because of patient or dental professional efforts at health maintenance. They intentionally may result in wear that eliminates excess material. Surfacing ones involve intentional abrading, finishing, and polishing during restorative procedures. Abrading is intentional reduction in surface height or volume, generally to flatten it. Finishing is an effort to smoothen a surface and often reduces surface irregularities to approximately 1 μ m sizes. Polishing is the removal of unwanted surface debris and/or further surface smoothening to reduce irregularities to the range of 0.1–1.0 μ m (Bayne et al. 2006; Bayne & Thompson 2006).
Table 9.2 Physiologic, pathologic, and prophylactic wear situations in dentistry.
In addition to wear categories and types, there are secondary effects from other things. The rate of loading (fast vs. slow) affects the material response (and apparent hardness). The loading level influences the number of fatigue cycles that the process may take to induce failure. The temperature alters the materials properties. The small range of intraoral temperature changes (5–60°C) may affect polymer phases, but will not affect metal or ceramic phases much at all.
The goals of this chapter are to use these definitions and terminology to assess biomaterials’ resistance to wear, consider the principles and concepts that govern wear rates, and determine the risk management necessary for successful use of biomaterials in different intraoral situations. Risk factors should allow any clinical situation to be judged as involving opportunities for short-term (0–5 years), medium-term (5–10 years), or long-term (10–20 years) success.
Properties versus structure
Any textbook (Bayne et al. 2004) on biomaterials science quickly points out that no material has a single value for each of its properties. Properties are determined by the structure of a material and that can vary for any composition. A quick review is the following: properties depend on the (a) arrangement (extent of crystallinity), (b) bonding patterns (types of primary and secondary bonding that might exist), (c) composition (number of phases, distribution of the phases, and sizes of the crystals), and (d) defects (point, line, area, and volume types). A change in the degree of crystallinity changes the properties. A change in crystallisation pattern changes the properties. A change in the concentration or distribution of line defects changes the properties. A change in the porosity present changes the properties. The last case is extremely important for dental materials. Manipulation that increases the porosity content by as little as 10% could effectively reduce the mechanical strength by as much as 40–50%. Most materials are strongly defect limited. Dental personnel have a major effect on defects present in final materials and thus on their properties. Therefore, the final wear resistance of any materials must be seen as operator dependent.
Principles and concepts for biomaterials wear
What follows in this chapter is an explanation of how different wear events are controlled by different intraoral circumstances. At first, however, consider the following overall principles and concepts:
- Operators: Operators are more important than materials. Significant variability exists among even good operators. Materials properties are defect limited (i.e. operator limited).
- Environmental factors: Failure/success of a biomaterial depends on environmental forces (physiologic, pathologic, prophylactic, and surfacing), extent of those cycles, intensity of those cycles, and time.
- Tooth locations: Intraoral tooth sites and intratooth surface locations determine the relative susceptibility to wear.
- Wear mechanisms: Different mechanisms at different tooth surface locations are affected differently by attrition, erosion, and abrasion.
On the basis of the above, one can already assess some biomaterials’ chances of survival. Is a good-to-great clinical operator involved? If so, almost anything will fare reasonably well. What is the risk status of the patient for caries? If the patient is one of the 20% considered high risk, all restorative materials outcomes will be limited in success. Does the patient display abnormal physiologic behaviors (e.g. bulimia and clenching)? Then both tooth structure and biomaterials will be significantly challenged. If the wear mechanism is countervailed by the material’s compositional design, then the material may wear very slowly over long time periods. Now, let us look at more details about each of these.
OVERVIEW OF BIOMATERIALS WEAR
Wear couples (wear assemblies)
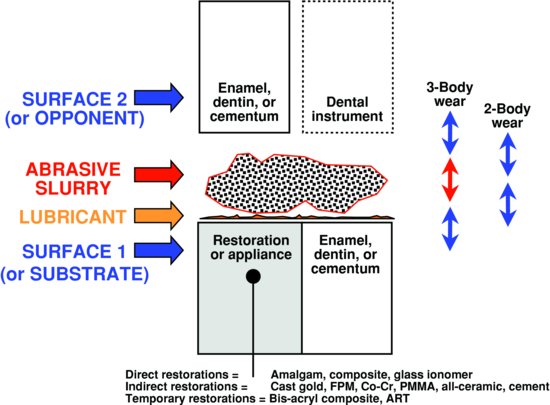
Understanding any local wear situation in dentistry requires identification of all the parts of the teeth/materials assembly (called the ‘wear couple’). Any assembly includes (a) a solid substrate surface (involving tooth structure [enamel, dentin, and cementum] and/or restorative materials [amalgam, composite, bonding agent, glass ionomer restorations, all-ceramic, porcelain fused to metal (PFM), gold alloys, stainless steel, prosthetic teeth, sealants, and/or atraumatic restorative technique (ART) materials]), (b) a solid opponent surface, (c) a lubricant (typically saliva), and/or (d) an interposed fluid phase (food, dentifrice, and polishing paste). This is schematically summarised in Fig. 9.2. This assembly is affected by the local temperature, pH, and intraoral functions. Wear is not a continuous event. It is cyclical depending on chewing cycles (loading cycles), pH cycles, or other physiologic rhythms. When the two surfaces are in direct contact with each other (even if a lubricant is present), the assembly is involved with two-body wear. If there is an interposed phase, then wear is called three-body wear.
Figure 9.2 Summary of all possible wear couples for teeth and restorations. The major components of substrate, opponent, lubricant, and abrasive slurry are shown. Two- and three-body wear are contrasted. The range of restorative materials is listed.
Once one knows the wear couple, it is possible to ask crucial questions about the wear event itself. What is the relative ‘potential’ for wear (high, medium, or low)? What is the typical ‘wear rate’ (i.e. kinetics or rate of the process [material loss/time])? What are the ‘microscopic events’ (i.e. wear mechanism)? How long will wear continue (i.e. extent of damage)? Each is dealt with in the following paragraphs.
Wear potential
A plethora of mechanical properties are associated with wear processes, but the key one is a material’s ‘hardness’. Material surfaces display elastic and plastic deformation in response to wear stresses. The hardness is the resistance to the onset of plastic deformation. Under a standard set of conditions, it is possible to rank comparative hardness using a variety of scales. Moh’s hardness scale is one of those scales and is simply the resistance to being scratched by comparison to a reference set of materials. A softer material gets scratched by a harder one. The reference materials are reported in Table 9.3 along with a range of dental materials examples.
As noted earlier, properties for a single composition can be quite variable, depending on the arrangement, bonding, compositional phases, and defects. Consider a couple of quick examples.
Table 9.3 Moh’s hardness values for reference materials and typical dental materials.a
| Reference material | Moh’s hardness value (1—10) | Dental material |
| Diamond, C | 10 | Cutting diamonds (10) |
| Yttria-stabilized zirconia (9–10) | ||
| Corundum, Al2O3 | 9 | Alumina (99) |
| Topaz, Al2SiO4(OH−,F−)2 | 8 | Cobalt–chromium alloys (7–8) |
| Quartz, SiO2 | 7 | Quartz polishing particles (7) |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


