9
Abutment Preparation Techniques for One-Piece Titanium and Zirconia Implants
1Washington Hospital Center, Department of Oral and Maxillofacial Surgery, Washington, DC; and American Institute of Implant Dentistry, Washington, DC
2Private Practice, Washington, DC
Introduction
The original Branemark protocol for the placement of dental implants was based on a two-piece implant and a two-stage procedure. Although this has been a predictably successful methodology, researchers have consistently worked to establish techniques to shorten the overall treatment time and improve esthetic outcomes while maintaining successful osseointegration. With the advent of advanced surface characteristics, the time needed for predictable osseointegration has shortened. As the concept of immediate loading has gained acceptance (Atieh et al. 2010; Tortamano et al. 2010; Den Hartog et al. 2011), the one-piece implant has become a viable option. The one-piece implant incorporates the implant body, a transmucosal element, and a restorative abutment into a single structure. As the abutment is present in the oral cavity from the time of placement, some degree of immediate loading is inherent. Studies continue to emerge that demonstrate that one-piece implants have a comparable survival rate to their traditional two-piece counterparts and that they can be a predictable restoration in implant dentistry (Borgonovo et al. 2010; Froum et al. 2011; Sohn et al. 2011).
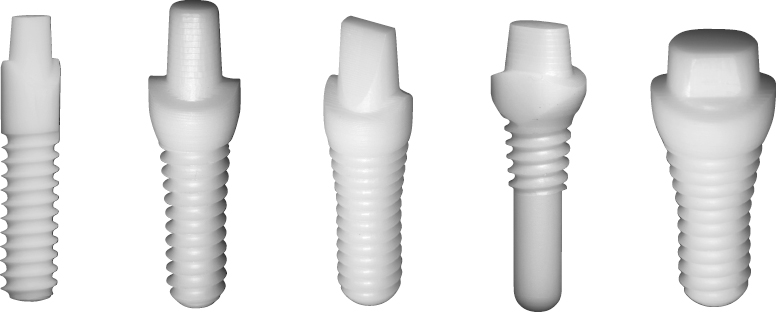
There are many advantages thought to be gained by the one-piece design. Because the internal implant abutment connective features are eliminated (Figure 9.1), small-diameter titanium implants as narrow as 3.0 mm can be manufactured without significantly increasing the risk of fracture (Allum et al. 2008). This has allowed appropriately sized implants to be used in sites that can be otherwise too narrow for conventional implant sizes, such as the mandibular incisor and maxillary lateral incisor areas (Figure 9.2).
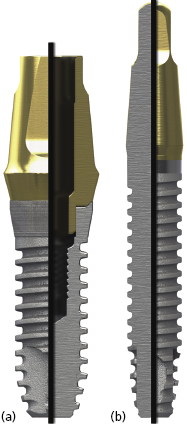
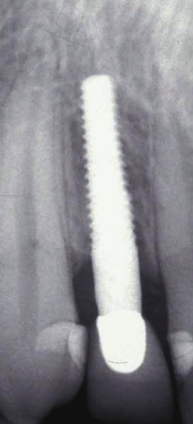
It should be noted that one-piece implants with less than a 3.0 mm diameter are produced – so-called “mini-implants” – but they are not categorized as permanent implants and are not recommended for the retention of crowns or dentures. They will not be the focus of discussion in this chapter.
With the elimination of the abutment screw, the one-piece titanium implant can actually be stronger than a two-piece counterpart of similar diameter (Allum et al. 2008). Additionally, without the micro-gap inherent to two-piece implant systems, the potential for micro-leakage leading to inflammation at the implant–abutment interface is eliminated. Further, the biologic width created around one-piece implants has dimensions closer to those of natural teeth than two-piece implants, which results in a more coronally located gingival margin (Hermann et al. 2001). As one-piece implants require a single-stage procedure, there is no need for a second surgery stage where manipulation of the crestal soft tissues and bone would usually occur. A second procedure will inherently induce inflammation and has the potential to precipitate crestal bone loss. Accomplishing all the surgical steps in one procedure also reduces the overall patient discomfort associated with treatment. Moreover, the lack of total component parts needed during treatment with a one-piece implant system can lower the overall cost incurred.
One-piece implants are currently manufactured from commercially pure (CP) titanium, titanium alloy, and zirconia. There is an ever-expanding number of companies producing one-piece implants; however only a few select designs will be covered in this text. The titanium designs discussed here have been chosen because they are part of the new generation of one-piece implants. The main distinguishing factor that separates the new generation is the inclusion of advanced surface characteristics on the implant body. These treatments, which produce increased protein binding to the implant surface, allow for immediate or early loading.
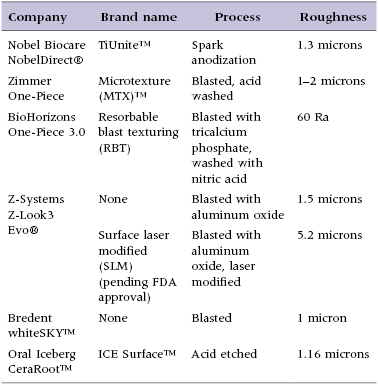
The new generation of titanium one-piece implants was introduced by Nobel Biocare with their NobelDirect® design, which utilizes their TiUnite™ surface. TiUnit is said to roughen the surface through a spark anodization process. Zimmer began producing a one-piece implant featuring their Microtexture MTX™ surface, produced by grit blasting the machined titanium implant surface with hydroxyapatite particles, followed by washing in non-etching acid and distilled water baths to remove the residual blasting material. BioHorizons also began producing a one-piece implant with a roughened surface from a blasting and acid-washing process known as the RBT (resorbable blast texturing) process.
In the new generation of titanium one-piece implants, developments have also been made in implant macro-geometry. Companies such as Zimmer and BioHorizons have enhanced the design of the abutment portion of the one-piece implant (Figure 9.3).
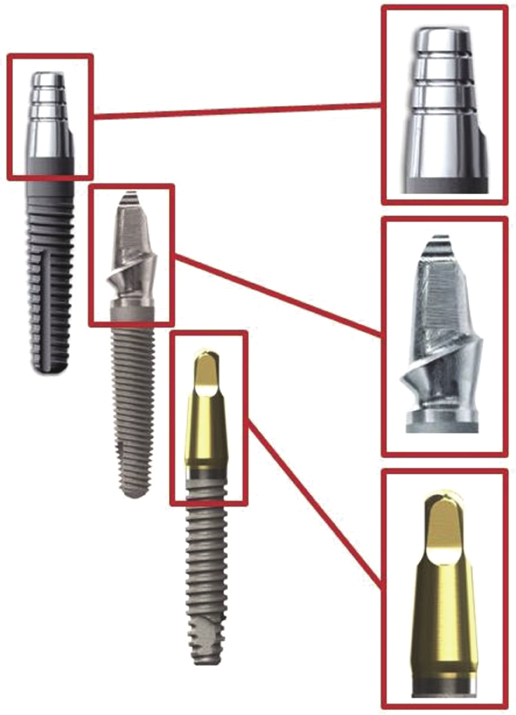
Zimmer introduced abutments with pre-prepared chamfer margins in order to decrease chairside preparation time and decrease heat generation at the implant surface. BioHorizons modified the abutment by including a titanium nitrite coating, which is thought to increase the esthetics of the final restoration by masking the dark hue of the titanium with a more natural color.
The zirconia one-piece implant designs covered in this text have been included because they were among the first uses of zirconia as an alternative to titanium in the implant dentistry. The Z-Systems Z-Look3 Evo® implant features a sand-blasted surface; however, the current European version of the implant utilizes a SLM (surface laser modified) surface. This process of blasting and laser etching is currently pending Food and Drug Administration (FDA) approval with plans to be available on the US market in late 2012. The Bredent whiteSKY™ implant also features a sand-blasted surface, while the Oral Iceberg CeraRoot™ implants are treated with an acid-etch process known as ICE Surface™. Since the surfaces currently available on zirconia implants do not qualify as advanced surface characteristics, they may have a slower increase in secondary stability and should be subjected to a protected load protocol during healing, as described later in the chapter.
One-piece implants have been shown to successfully osseointegrate, have good long-term survival rates, and have produced excellent esthetics in multiple clinical studies (Borgonovo et al. 2010; Froum et al. 2011; Sohn et al. 2011). In order to understand the clinical success of the one-piece implant it is important to analyze their geometry at the macro- and micro-level, as well as their indications for use and the proper surgical technique required for their placement.
Material Science of One-Piece Implants
Zirconia originated in 1789 as the discovery of German chemist M. H. Klaproth. However, it was only introduced into dentistry a few decades ago: a product of the increasing desire for highly esthetic restorations. Zirconia has found many applications in dentistry, but has only recently become a prominent figure on the implant dentistry stage. Other ceramics, such as aluminum oxide, were used in the early stages of implant dentistry and were able to osseointegrate and provide esthetic restorations, but did not have the mechanical strength to withstand long-term bite forces. These early ceramics fell out of favor due to their shortcomings and titanium became the mainstay for implant manufacturing.
With recent advances in the chemical processing of zirconia, it has become the first ceramic material in dental implantology able to withstand long-term loading, and subsequently has become an attractive alternative to titanium (Kohal et al. 2002, 2011; Silva et al. 2009). Zirconia has a white hue that can effectively mimic the shade of natural teeth and eliminate the unsightly gingival discoloration often encountered with titanium implants in the esthetic zone. Furthermore, zirconia has not only been shown to have similar bone–implant contact percentages as titanium (Koch et al. 2010; Stadlinger et al. 2010), but also a lesser degree of bacterial adhesion and plaque accumulation. This may have implications for the likelihood of developing peri-implantitis (Scarano et al. 2004). Zirconia has also been noted to foster excellent soft tissue attachment due to its strong biocompatibility (Stadlinger et al. 2010). Based on these properties, zirconia has demonstrated its utility as a viable alternative to titanium.
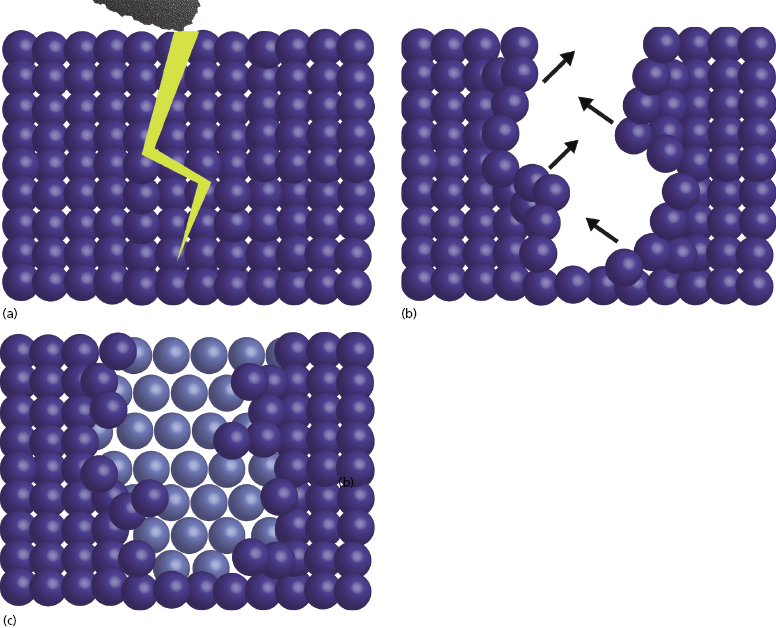
The zirconia used in dentistry today is not merely the zirconium dioxide discovered in the 18th century; commercial grade zirconia has several modifications that enhance its properties. In its pure phase, zirconium dioxide has a low shear strength and is very brittle, essentially making it useless as a dental material. The addition of small amounts of aluminum oxide and yttrium oxide increase the modulus of elasticity and help to stabilize the material. When this combination is mixed with zirconium oxide in the powder state and placed in a sintering oven, a monocline crystalline structure is produced (Figure 9.4a).
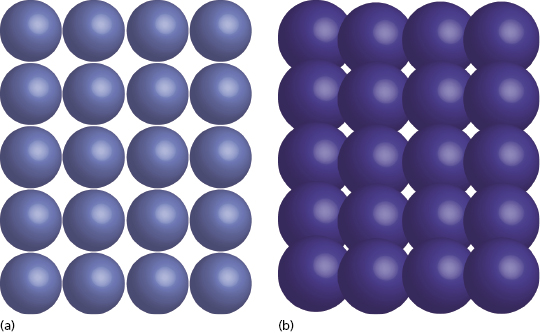
For crown and bridge dentistry, restorations are milled from zirconia blanks and then sintered to produce this monocline structure. Although the monocline crystal is a quite strong, when cracks occur they propagate easily through the structure. As the zirconia used for implant dentistry often requires preparation with a high-speed handpiece, there is a potential to cause small cracks in the material surface. Over time, these could progress and eventually lead to material failure. This property makes monocline zirconia less desirable to use as a long-term implanted prosthesis.
In order to eliminate this issue, the zirconia used for dental implants is also exposed to a process known as HIP, or hot isostatic pressing. The high pressure under which the monocline zirconia is placed during HIP processing causes condensation of the particles and results in a tetragonal crystalline structure (Figure 9.4b). The significance of this innovation is that it imparts the ability to impede crack propagation. When the surface of HIP-processed zirconia is prepared, any micro-cracks that may result are quickly stabilized as tetragonal particles expand within the monocline structure to fill the void. This self-repairing property is also known as the “airbag effect” (Figure 9.5).
In order to manufacture zirconia dental implants, HIP-processed tetragonal zirconia blanks are milled into the desired implant shape (Figure 9.6).
The additional stability gained by the HIP process has enabled zirconia to be used for multiple medical prosthetics with lifespans comparable to their metal-based counterparts (Kohal et al. 2002, 2011; Silva et al. 2009).
One-Piece Implant Macro-Geometry
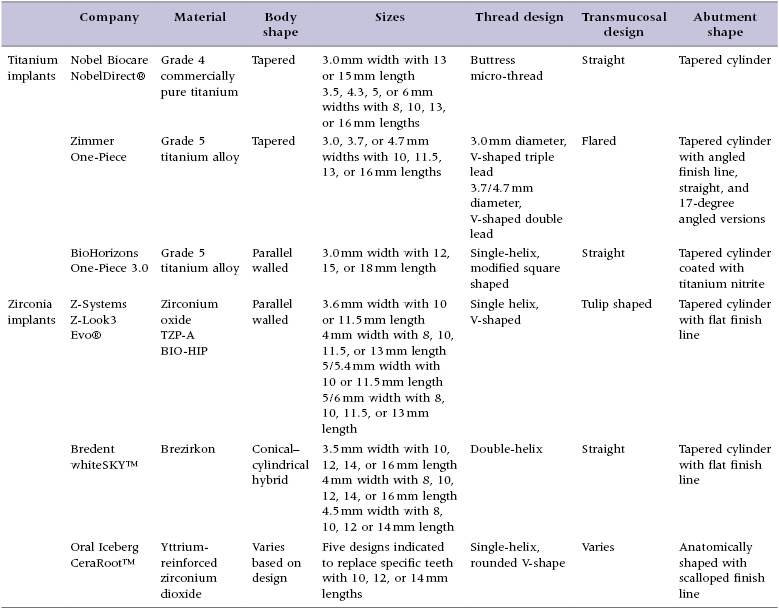
Implant macro-geometry includes features such as the material used, shape of the implant body, length and width of the implant, and thread design, as well as the overall shape of the transmucosal element and restorative abutment. These elements are examined in detail as they pertain to the implant designs reviewed in this text to allow the clinician to gain an appreciation for the design features available (Table 9.1).
Table 9.1 Comparison of the design elements of selected one-piece implants
Nobel Biocare
Nobel Biocare NobelDirect implants were introduced in March 2004. The implants were originally produced with a 3.0 mm diameter with either a 13 or 15 mm length, known as NobelDirect 3.0 (Figure 9.7a).

Later, Nobel Biocare also produced one-piece implants with 3.5, 4.3, 5, and 6 mm diameters with 8 to 16 mm lengths, known as NobelDirect Groovy, Oval, and Posterior. Although still available, these designs will not be the focus of this chapter.
The NobelDirect 3.0 implants are produced from cold-worked grade 4 CP titanium. The implant body has a tapered design with a 2.0 mm diameter at the apex and is treated with Nobel Biocare’s TiUnite surface. The implant features a buttress micro-thread with a 0.6 mm thread pitch. The NobelDirect 3.0 implant has a straight transmucosal element which is 3 mm in height, measured from the most coronal aspect of the threaded portion to just above the apical extent of the machined surface. This area, up to the beginning of the polished portion, is also treated with the TiUnite surface. The rationale for roughening the surface of the transmucosal portion was to create a stronger soft tissue seal, based on literature that suggests a shorter contact epithelium is created at oxidized, acid-etched surfaces (Glauser et al. 2005). The abutment portion of the implant is 5 mm in height and has a tapered cylindrical shape with one flattened portion to act as an antirotational element. The abutment is manufactured with a smooth, machined surface and does not have a predetermined restorative finish line.
Zimmer
The Zimmer One-Piece implant, introduced in 2007, is produced from grade 5 titanium alloy (Figure 9.7b). The implants are produced in 3.0, 3.7, and 4.7 mm diameters with lengths ranging from 10 to 16 mm. The implant body has a tapered design, with shorter implants having larger degrees of taper. The 3.0 mm diameter implants have a triple-lead V-shaped thread design, whereas the 3.7 and 4.7 mm diameter implants are given a double-lead V-shaped thread. The entire implant body is treated with the MTX surface, which includes the threaded portion plus an unthreaded area at the coronal aspect. A 0.5 mm machined collar forms the base of the transmucosal element. The transmucosal area of the implant has a flared design and is 1.2 mm in height at the buccal margin. The flared design is thought to allow greater tissue thickness circumferentially at the alveolar crest to mask the implant’s gray hue and to produce a more natural emergence profile. The implants are produced with either straight or 17-degree angled abutments, which are 4–6 mm in height for the 3.0 mm diameter implants (Figure 9.8).

The abutments are manufactured with a pre-established chamfer finish line. The finish line is angled along the circumference of the abutment, with the more apical aspect corresponding with the facial crown margin. The purpose of including the pre-established finish line was to decrease the need for chairside preparation, which is thought to produce heat and damage the adjacent bone, potentially compromising osseointegration. For the 3.0 mm diameter implants, the restorative platform has 3.5 mm diameter.
BioHorizons
BioHorizons introduced the BioHorizons One-Piece 3.0 implant (formerly Maximus) in December 2003 (see Figure 9.7c). The implants are produced from grade 5 titanium alloy and are only produced in a 3.0 mm diameter with 12, 15, or 18 mm lengths. The majority of the implant body is parallel-walled with a tapered apical portion. The implants feature a modified square-shaped thread design (Figure 9.9).

The implant body is treated with their RBT surface. The implant has a predominantly straight transmucosal element with a very subtle flare at the transition to the titanium nitrite-treated surface. There is a 0.7 mm machined collar just coronal to the first thread. The abutment has a tapered cylindrical shape without a restorative finish line. The abutment portion is 8 mm in height and coated with a 3-micron thick titanium nitrite coating, giving it a gold hue. This surface coloration is intended to mask the titanium with a more natural color to improve the appearance of the final restoration and decrease gingival discoloration, particularly in those with a thin gingival biotype. The coronal aspect of the abutment has a narrowed portion with four flattened surfaces, which allows a stable connection between the implant and driver during placement.
Z-Systems
Implants from Z-Systems have been available in Europe since 2004 and the Z-Look3 implant, their original design, was approved for use on the American market in 2007. Z-Systems’ second generation implant, the Z-Look3 Evo (Figure 9.10a), became available in the USA in September 2011 and included changes to the implant sizes offered and the design of the abutment portion.
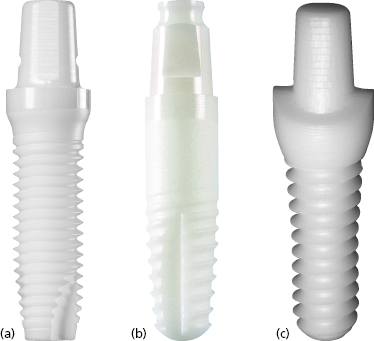
The original design included an insertion hex above the abutment portion which had to be ground off following placement. This element was eliminated in the second generation design to minimize the amount of preparation required for the newly placed implants. Instead, a retention groove along the side of the abutment was added. Z-Systems implants are produced from zirconia TZP-A BIO-HIP®, which is a HIP-processed zirconia. The Z-Look3 Evo implants are available in various sizes: 3.6 mm diameter with 10 and 11.5 mm lengths, 4 mm diameter with 8, 10, 11.5, and 13 mm lengths, 5 mm diameter with 5.4 mm restorative platform in either 10 or 11.5 mm lengths, or 5 mm diameter with 6 mm restorative platform in 8, 10, 11.5, and 13 mm lengths. The implant body has a parallel-walled design with an apical taper and a single-helix V-shaped thread design. The surface of the implant body is treated with a sand-blasting process, and the total length of the sand-blasted portion of the implant corresponds to the advertised implant length. As previously mentioned, a new surface treatment known as SLM is currently pending FDA approval and will be incorporated into the design when approved. The transmucosal area has a “tulip shape,” which is essentially a convex, rounded flare that leads up to the restorative shoulder. The implant restorative abutment area has a tapered cylindrical shape with a flattened area that acts as an antirotational element during insertion. The implant shoulder is symmetric with a flat restorative finish line.
Bredent
The Bredent whiteSKY implant (Figure 9.10b) was brought to the European market in April 2006, but is not currently available in the USA. It is manufactured from Brezirkon®, Bredent’s yttrium oxide-stabilized, tetragonal polycrystalline zirconium oxide. Implants are produced with 3.5, 4, and 4.5 mm diameters with lengths ranging from 8 to 16 mm. The implant body has a conical–cylindrical hybrid form, meaning that the threaded apical portion of the implant body is tapered, while the non-threaded coronal 3 mm has a cylindrical shape. The threaded portion of the implant body has a double-helix thread design, and both the unthreaded and threaded portions of the implant body have a sand-blasted surface. The implant length is measured from the apex of the implant to the coronal edge of the sand-blasted surface. The transmucosal aspect of the implant is 2 mm in height and has a straight design with a smooth, machined surface. The abutment portion of the implant is 6.8 mm in height and has a tapered cylindrical shape with a flattened side that acts as an antirotational element. There is a circumferential groove at the most coronal aspect of the abutment, which serves to stabilize the implant within its packaging. The restorative finish line has a flat design with a restorative platform that corresponds to the implant body width for the 4.0 and 4.5 mm diameter implants. For the 3.5 mm diameter implant, the transmucosal component of the implant is slightly wider than the implant body, allowing for a 4.0 mm restorative platform at the finish line.
Oral Iceberg
Oral Iceberg is the manufacturer of CeraRoot implants (see Figure 9.10c), a unique line of yttrium-reinforced zirconium dioxide implants that were brought to the European market in 2007. They received FDA approval in January 2011 and are now being marketed in the USA. The CeraRoot system features five different implant designs (Figure 9.11), each meant to be particularly well suited for the replacement of specific teeth within the arch.
The CeraRoot 12 design is a 4.1 mm diameter implant with a threaded, parallel-walled implant body meant for use in mandibular incisor and maxillary lateral incisor sites. The CeraRoot 11 and 21 implants are similar in design; both have implant bodies with parallel-walled apical portions and flared, conical coronal portions. CeraRoot 21 implants are intended to replace maxillary central incisors as well as maxillary and mandibular canines; they feature a 4.1 mm implant body diameter at the apex and a 6 mm diameter restorative platform. CeraRoot 11 implants, on the other hand, are only meant to replace maxillary central incisors and canines, and have a 4.8 mm diameter at the apex and a 6.5 mm restorative platform. CeraRoot 16 implants have a shape similar to the 11 and 21 designs with a cylindrical implant body at the apex but flared design in the coronal aspect; however, they have a wider diameter to accommodate molar sites. The implant body has a 4.8 mm diameter at the apex with an 8 mm diameter restorative platform.
The CeraRoot 14 design is quite different from the other designs. The implant has an oval cross-section at the level of the restorative platform, meant to replicate the normal cross-section of a premolar tooth. This more anatomic shape is meant to provide the best emergence profile and overall esthetic result for the replacement of premolars. Due to the non-traditional shape of this implant, it is placed with a press-fit technique, as described later in the text. For this reason, the apical portion of the implant body is not threaded, and threading on the coronal portion of the implant body is only present to further stabilize the implant during initial placement and healing.
All CeraRoot designs are available in 10, 12, or 14 mm implant body lengths. The thread design for the CeraRoot implants is a single-helix, rounded V shape, and the implant surface is treated with an acid-etching process known as ICE Surface. The transmucosal element of the implant, which is defined as the area between the most coronal thread and the most apical portion of the finish line, ranges in height from 2.5 to 3.5 mm depending on the implant design. For CeraRoot 12 implants, the transmucosal portion is straight, while for CeraRoot 11, 21, and 16 is takes on a flared shape. In CeraRoot 14 implants, the transmucosal design may be described as more of a tulip shape. The abutment portions of the CeraRoot implants were designed to mimic the shape of a traditional crown preparation. Thus, the implants meant for incisor sites have incisor crown preparation-shaped abutments, while the molar implants have molar crown preparation-shaped abutments. Each has at least one flattened surface that acts as an antirotational element. The restorative finish lines are also meant to correspond to the ideal shape of traditional crown preparations. The restorative finish lines are scalloped, with the most apical portions designed to be positioned on the buccal and lingual aspects of the surgical site, while the most coronal portions of the finish line are to be placed in the mesial and distal positions.
One-Piece Implant Micro-Geometry
Micro-geometry in implant dentistry refers largely to the surface texture of the implant body. Having a roughened implant surface is known to enhance migration and proliferation of osteoblasts and to precipitate osseointegration (Nasatzky et al. 2003; Osathanon et al. 2011). Ideal surface roughness is thought to be in the range of 1–100 microns for the best osteoblast attachment, which ultimately results in the highest bone–implant connection. Blasting processes, which are used by multiple companies, serve to increase the surface area of the implant body through a subtractive process, resulting in peaks and valleys across the implant surface. Often the implant surfaces are also treated with an acid. Varying terminology exists for these processes, such as acid washing and acid etching. It should be noted that washing with an acid also results in etching the surface, so the terminology is essentially equivocal. Treatment with an acid will remove residual debris from a previously blasted implant surface in addition to etching the surface.
These processes result in a decreased contact angle, which allows better fluid flow and protein binding along the implant surface. These processes all serve to increase bone–implant contact percentages and overall stability of the implants, which ultimately results in better long-term survival. The prominent features of the micro-geometry of the designs covered in this chapter are presented in Table 9.2.
Table 9.2 Comparison of the micro-geometry of selected one-piece implants
The TiUnite surface applied to Nobel Biocare NobelDirect implants is an electrochemical oxidation procedure which Nobel Biocare began applying to their implants in 2000. It results in a surface with a porous texture and a roughness of approximately 1.2 microns (Figure 9.12a) (Schuepbach and Glauser 2012).
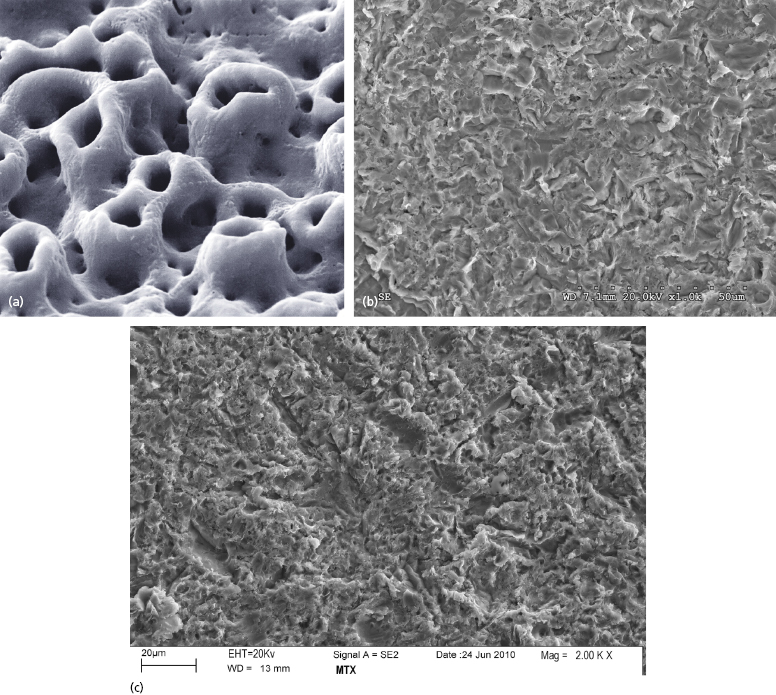
The BioHorizons One-Piece 3.0 implants are treated with their RBT process, which involves blasting the implant surface with tricalcium phosphate, followed by acid washing. This results in a surface roughness of approximately 2 microns (Figure 9.12b). Zimmer One-Piece implants have surfaces treated with their MTX process, in which the implant surface is blasted with hydroxyapatite particles, then washed in non-etching acid and distilled water baths to remove the residual blasting material. The MTX process results in a surface roughness of approximately 1–2 microns (Figure 9.12c).
As zirconia dental implants are newer to the market, the technology available for surface treatments is somewhat less sophisticated as compared with titanium at this time. Both Z-Systems Z-Look3 Evo and Bredent whiteSKY implants utilize a sand-blasting process to treat the zirconia surface (Figure 9.13a, b).
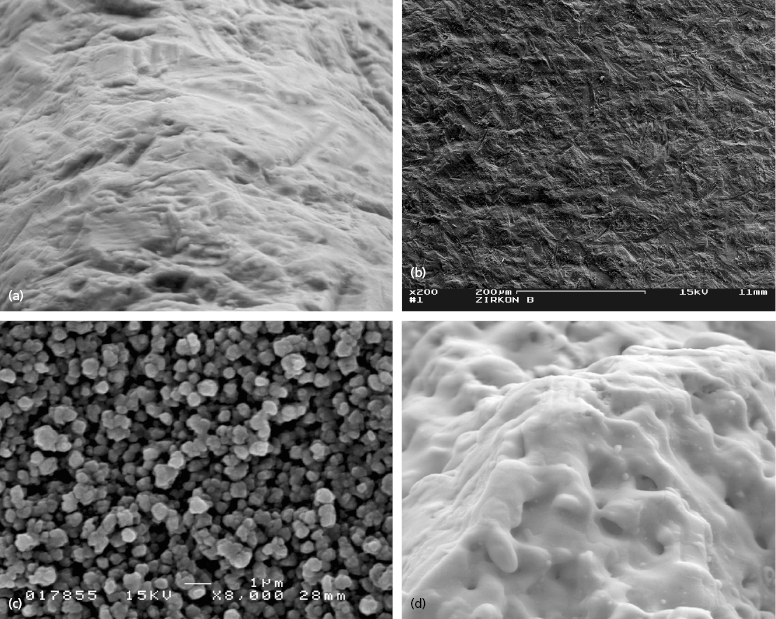
These processes result in a surface roughness of approximately 1.5 and 1 micron, respectively. The Oral Iceberg CeraRoot implants, on the other hand, are now utilizing an acid-etching process to treat the implant surface known as the ICE Surface (Figure 9.13c). The Z-Systems implants recently introduced a new FDA-approved surface characteristic known as SLM. This process consists of blasting the implant surface, then further modifying the roughness through the use of a laser (Figure 9.13d).
Case Selection Criteria
General Considerations
Patient compliance is especially important for success in one-piece implants. The patient must be able to avoid chewing with the provisional crown or religiously wear their removable protective appliance during healing so that unfavorable forces on the implant can be limited.
Anatomic Considerations
Three-Dimensional Space Limitation
One-piece implants have been designed that can be used to replace any tooth. However, their most unique application is for narrow spaces in which traditional implant sizes would surpass the limits of the bone volume (Figures 9.14 and 9.15).
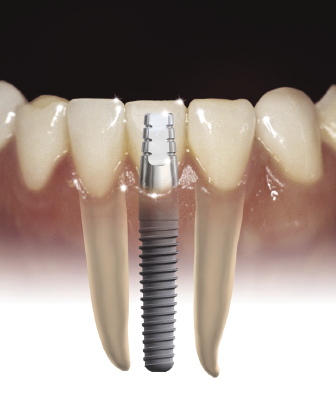
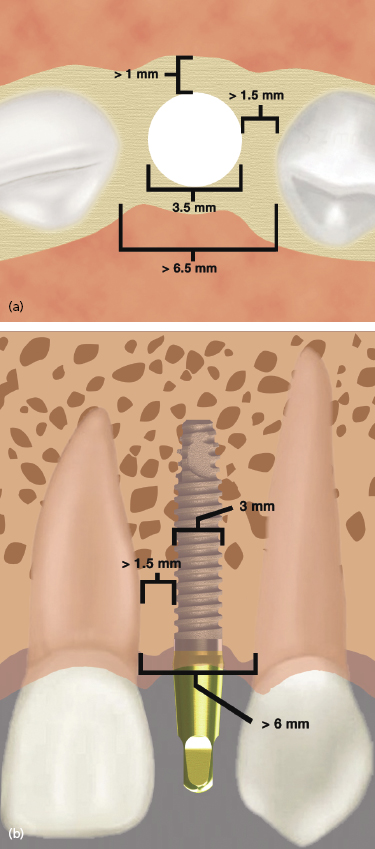
Often the buccolingual and mesiodistal dimensions of the alveolus are reduced in the maxillary and mandibular incisor areas, and placement of a standard-sized 3.5 or 4 mm diameter implant can lead to the exposure of implant threads and bone loss or damage to adjacent teeth (Sohn et al. 2011). Due to the one-piece design and greater internal bulk of material, titanium one-piece permanent implants can be manufactured with very small diameters while still maintaining excellent strength. Two-piece implants are also currently being manufactured with 3.0 mm diameters, but will inherently have a greater risk of fracture due to their design.
The narrow-diameter implants are indicated for mandibular incisor and maxillary lateral incisor sites. They are not recommended for use in posterior sites with small mesiodistal lengths where traditional implants sizes cannot be used, or generally for the replacement of premolar or molar teeth. By using these narrow-diameter implants for the replacement of incisors, teeth with small cervical diameters can be replaced with implants of a similar diameter, which helps to achieve the best emergence profile and esthetic result. Implants t/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


