Supportive Implant Treatment
Rationale for Supportive Implant Treatment
Although dental implants are not vulnerable to dental caries, they are still susceptible to mechanical complications and periimplant plaque-induced inflammatory tissue changes. A 10-year retrospective study48 of 397 fixed-implant reconstructions in 300 patients observed a mechanical complication rate of 24.7%. The most frequent complication was ceramic chipping (20.31%) followed by occlusal screw loosening (2.57%) and loss of retention (2.06%). Although relatively infrequent, occlusal screw loosening can result in a subgingival gap at the implant-abutment junction that retains plaque and stimulates an inflammatory reaction in both the soft and hard tissue.
Biologically, periimplant plaque accumulation due to inadequate or lack of access for oral hygiene can result in periimplant mucositis and periimplantitis. Periimplant mucositis is characterized by inflammation confined to the soft tissue and is reported to affect up to 80% of patients with dental implants.25 Periimplantitis is characterized by periimplant inflammation with progressive crestal bone loss beyond the initial remodeling. The prevalence of periimplantitis in patients ranges from 11.2% to 53%.17,20,27,37,38,39,40 Poor oral hygiene, residual cement, current or history of periodontitis, cigarette smoking, and diabetes mellitus are risk factors for periimplant diseases.1,9
The relationship between periimplant mucositis and peri-implantitis is similar to that of gingivitis and periodontitis. Although periimplant mucositis does not necessarily progress to periimplantitis, it is likely the precursor to periimplantitis.1 The inflammatory response in periimplant disease appears to be similar to that in periodontal disease.41 However, severity and rate of disease progression appear to be more pronounced around implants. This, perhaps, is due to the absence of a “self-limiting” process, observed in periodontitis, around implants that separates the inflammatory cell infiltrate from the bone.6 Experimental models demonstrated that periimplant mucositis is reversible at the biomarker level (matrix metalloproteinase 8 [MMP-8] and interleukin-1β [IL-1β]).41 A review of the literature36 reported that periimplant mucositis can be effectively treated with nonsurgical mechanical therapy, while such modalities tend to be ineffective against peri-implantitis. Furthermore, results of surgical treatment for peri-implantitis are not predictable. For these reasons, the prevention, early detection, and early treatment of periimplant diseases are critical. Periodic and well-regimented supportive implant treatment is essential to the long-term success of dental implant therapy.
Examination of Implant(s)
Examination begins with visual inspection for: plaque and calculus accumulation; signs of inflammation and swelling; peri-implant soft tissue quality, color, consistency, and contour; and aberrations in the implant prostheses. Periimplant soft tissue can be digitally palpated to detect edema, tenderness, exudation, or suppuration. Periimplant probing can be used to assess the condition and level of soft and hard tissues surrounding implants. When indicated, radiographic images can be obtained to help verify the level of the periimplant crestal bone. Determination of implant stability (mobility) and percussion testing can help verify implant osseointegration. Figure 83-1.
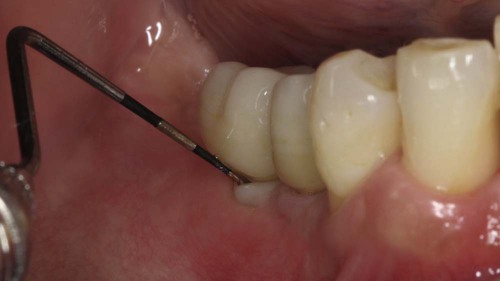
Periimplant Probing
Probing of implants can be done with light force (0.25 N) using a traditional steel probe without adverse effects to the periimplant mucosa.15 Implant probing should be recorded at the time of final restoration delivery as the baseline measurement and at least annually thereafter.25
Clinicians should use caution when evaluating periimplant probing because these measures cannot be interpreted the same as probing depths around teeth. While periodontal probing around natural teeth is very useful to assess the health of periodontal tissues, the sulcus or pocket depth, and the level of attachment, probing around implants may not provide comparable results.7 Due to distinct differences in the tissues that surround and support teeth compared to those that surround and support implants, the probe inserts and penetrates differently. Around teeth, the periodontal probe is resisted by the health of the periodontal tissues and, perhaps most importantly, by the insertion of supracrestal connective tissue fibers into the cementum of the root surface. These fibers, unique to teeth, are the primary source of resistance to the probe.3 There is no equivalent fiber attachment around implants. Connective tissue fibers around implants generally run parallel to the implant or restorative surface and do not have perpendicular or inserting fibers (see Chapter 71). The primary source of resistance to the probe around an implant will differ depending on the conditions surrounding the implant.12,24 At noninflamed sites, the most coronal aspect of connective tissue adhesion to the implant will resist the probe. At inflamed sites, the probe tip consistently penetrates farther into the connective tissue until less inflamed connective tissue is encountered, which is often close to or at the level of bone.
The value of periimplant probing is different than periodontal probing and offers very limited information by comparison. Probing around implants can measure the level of the mucosal margin relative to a fixed position on the implant or restoration and can also measure the depth of tissue around the implant. The peri-implant probing depth is often a measure of the thickness of the surrounding connective tissues and correlates most consistently with the level of surrounding bone. However, periimplant probing is affected by several conditions, including the size of the probe, the force and direction of insertion, the health and resistance of periimplant tissues, the level of bone support, and the features of the implant, abutment, and prosthesis design (Figure 83-2). A comparison45 of probing pocket depth of implants with periimplantitis before and after the removal of the prosthetic restorations reported similar probing depths in only 37% of sites. In 39% of sites, the difference was ±1 mm, in 15% of sites, it was ±2 mm, and in 9% of sites, it was ±3 mm. In other words, probing measurements can be an accurate measure of soft tissue thickness around an implant (i.e., periimplant soft tissue above the bone level), but in many cases or sites, the inability to properly angle and direct the probe alongside of the implant may lead to inaccurate assessment of soft tissue thickness. In these situations, the clinician must appreciate the limitations and know that other clinical parameters and radiographs are required to help evaluate periimplant condition. Furthermore, probing around implants is likely to be more variable than around teeth; studies have shown that a change in probing force around implants results in more dramatic changes than a similar change in probing force around teeth.29 The probing depth around implants presumed to be “healthy” (and without bleeding) has been documented to be about 3 mm around all surfaces.2,8 The absence of bleeding on probing around teeth has been established as an indicator of health and a predictor of periodontal stability.21 Studies comparing bleeding on probing around teeth and implants in the same patient have reported that bleeding around implants occurs more frequently. The presence of bleeding on probing at implant sites may indicate inflammation in the periimplant mucosa. However, the ability to use bleeding on probing as an indicator of assessing diseased versus healthy sites around implants has not been established. Due to the potential for false-positive bleeding (i.e., provoked-bleeding) with probing, the use of marginal bleeding, which is a more sensitive indicator of inflammation and is less likely to elicit false-positive bleeding, has been proposed to assess periimplant inflammation.50 Marginal bleeding can be evaluated by running a probe circumferentially along the coronal portion in the implant sulcus. Overall, the value of periimplant probing is in monitoring changes in the probing pocket depth over time rather than the initial value as some implants may be placed apically for esthetics.1
Microbial Testing
Studies in animals and humans have demonstrated the development of periimplant mucosal inflammation in response to the accumulation of bacterial plaque.5,33,41,51 Microbiologic studies suggest that greater probing depths or “pockets” around implants harbor higher levels of pathogenic microorganisms.30,35,43 Studies have also documented similarities in the microbial composition of plaque in healthy periodontal sites compared to healthy peri-implant sites.31 Likewise, evidence indicates that the microbiota of diseased periodontal pockets harbor the same periodontal pathogenic microorganisms as those observed in inflamed periimplant sites (periimplantitis).31,43 However, there is no evidence to prove that periodontal pathogens cause periimplant disease, and the pathogenesis of inflammatory disease around implants has not been defined.10 A recent report1 now generally accepts the belief that periimplant disease, like periodontal disease, occurs primarily as a result of an overwhelming bacterial insult and subsequent host response. Furthermore, human biopsies indicate that periimplantitis and periodontitis exhibit similar histologic features including an inflammatory cell infiltrate in the connective tissue dominated by B-lymphocytes and plasma cells as well as upregulation of inflammatory biomarkers. No convincing evidence indicates that laboratory tests for the identification of suspected periodontal pathogens are of any use in the evaluation of implants.14 The usefulness of microbial testing may be limited to the evaluation of periimplant sites that are showing signs of infection and bone loss, so the clinician can prescribe appropriate antibiotics.
Stability Measures
There is great interest in evaluating the stability of the bone-to-implant contact in a noninvasive manner. Two noninvasive techniques that have been used for evaluating implant stability are impact resistance (e.g., Periotest) and resonance frequency analysis (RFA). Originally designed to evaluate tooth mobility quantitatively, the Periotest (Gulden, Bensheim, Germany) is a noninvasive, electronic device that provides an objective measurement of the reaction of the periodontium to a defined impact load applied to the tooth crown. The Periotest value depends to some extent on tooth mobility but mainly on the dampening characteristics of the periodontium. Despite the dependence on the periodontium, the Periotest has been used to evaluate implant stability as well. However, unlike teeth, the movement of implants and the surrounding bone is minuscule, and therefore the Periotest values fall within a much smaller range compared to the range found with teeth. Detection of horizontal mobility may be a significant advantage for the use of the Periotest because it is much more sensitive to horizontal movement than similar detection by other means, such as manual assessment.14 Another noninvasive method used to measure the stability of implants is with RFA.28 This method uses a transducer that is attached to the implant or abutment. A steady-state signal is applied to the implant through the transducer, and a response is measured. The RFA value is a function of the stiffness of the implant in the surrounding tissues. The stiffness is influenced by the implant, the interface between the implant and bone and soft tissues, as well as the surrounding bone itself. Additionally, the height of the implant or abutment above the bone will influence the RFA value. Unlike the Periotest, however, the RFA is not dependent on movement in only one direction. Thus the absolute RFA values vary from one implant design to another and from one site to another, but there is high consistency for any one implant or location. The value of RFA is most appreciated with repeated measures of the same implant over time because it is very sensitive to changes in the bone-implant interface. Small changes in tissue support can be detected using RFA. An increase in RFA value indicates increased implant stability, whereas a decrease indicates loss of stability. However, this is a relative measure and it has not been determined whether RFA is capable of detec/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses