Chapter 6
Pathological Aspects of Oral Precancer
Oral epithelial dysplasia is the histopathological term used to describe a spectrum of tissue dysmaturation and disorganisation changes, involving both architectural disturbance and cytological atypia, seen in biopsy specimens taken from oral potentially malignant lesions. The presence of such dysplastic regions within oral epithelium is thought to be associated with an enhanced possibility of malignant transformation, with the most severe dysplasias probably at greatest risk.
The identification and subsequent grading of the severity of dysplastic change is thus fundamental to oral precancer diagnosis and its subsequent management. The relationship and communication between the clinician in the dysplasia clinic and the pathologist in the laboratory, therefore, is absolutely central to accurate diagnosis, clinical decision making, treatment planning and ultimately, of course, the overall welfare of oral potentially malignant disorder patients.
Interpreting the significance of clinically observed oral mucosal lesions requires tissue samples to be obtained by biopsy and transported to the laboratory for specialised histopathological assessment and diagnosis. Although molecular biology is being increasingly used in oncology research, traditional light microscopic assessment of formalin-fixed tissue remains the cornerstone of diagnostic pathology and is likely to remain so for many years to come.
In this chapter we will explore the fundamental principles of pathological science as applied to the diagnosis and management of oral precancer.
Accurate pathological diagnosis is reliant on appropriate sampling of the oral potentially malignant lesion. In Chapter 5, we discussed the diagnostic process for potentially malignant disorders and emphasised the fundamental importance of biopsy. It is vital that the clinician selects the most appropriate sites for biopsy and, on occasion, multiple samples may be required to map widespread field change.
Except for very small lesions, it is usual to perform incisional biopsies first to obtain a tissue diagnosis. It is well recognised that invasive carcinoma may be diagnosed in unexpected situations; examples include flat white or red mucosal patches and erythematous gingival lesions. Consequently biopsy of any suspicious, non-healing or unexplained oral lesions is mandatory.
Incisional biopsies of potentially malignant lesions must be adequate in size and of sufficient depth to include the reticular lamina propria. Achieving sufficient depth can be a problem with thick keratinising lesions. Elliptical scalpel biopsies have been used for many years but there is an increasing preference for the punch biopsy [1]. Punch biopsy instruments are available in a range of diameters up to 10 mm. Before taking the biopsy, local analgesia should be achieved by injection around (but not directly into) the mucosal area to be sampled. After making a circular incision the punch biopsy can be released with minimal trauma using scissors.
The biopsy should be placed immediately into at least ten times its volume of fixative. Normally the laboratory providing the diagnostic service will provide pre-filled biopsy containers with advice on labelling, transporting to the laboratory and other issues to ensure an effective and safe service.
From the point of view of the pathologist, mucosal punch biopsies offer many advantages. Standard operating procedures can be devised to ensure a consistent approach in the pathology laboratory to specimen trimming, biopsy orientation, embedding and sectioning with preparation of appropriate levels. A more rapid turnaround time is facilitated and problems with interpretation of poorly orientated biopsies can be minimised.
In oral potentially malignant lesions the interface between the oral epithelium and lamina propria can be complex, particularly in thick lesions. Adverse orientation of sections can exaggerate the complex architecture and lead to difficulties in recognising whether invasion is present or not. Punch biopsies have been reported to cause less morbidity than scalpel biopsies and can be closed with a single suture or left to heal by primary intention. In a volunteer study to assess tolerance of 3 mm buccal punch biopsies, all subjects stated that they would be willing to undergo a subsequent biopsy procedure [2].
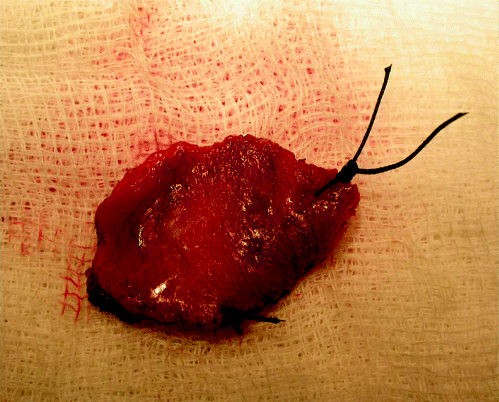
A limitation of even multiple punch biopsy evaluation is sampling. One powerful argument favouring laser excision of oral potentially malignant lesions is that the entire clinical lesion can be sampled. Sometimes this leads to a diagnosis of invasive carcinoma that was not suspected clinically. Standard operating procedures for trimming laser excisions should be used in the pathology laboratory. The specimen should be accompanied by a diagram or photograph and orientation sutures can be inserted. Figure 6.1 demonstrates a laser excision specimen immediately following surgery with orientation sutures in situ to distinguish the anterior and lateral excision margins. Mucosal margins can also be inked. Serial blocking techniques are most effective and good clinical correlation is needed to ensure clarity of diagnosis in relation to margin status.
Figure 6.1 Laser excision specimen showing orientation sutures at the anterior and lateral excision margins.
A large body of literature has accumulated describing molecular alterations in pathways that control cellular signalling, cytoskeleton development, cell cycles, apoptosis, genomic stability and angiogenesis, amongst others. Many of these studies suffer from cross-sectional design, a relatively small sample size and a lack of consistent diagnostic criteria. No biomarkers are currently used in routine practice for the evaluation of oral potentially malignant disorders. A promising approach is combined analysis, for example the detection of aneuploidy with other biomarkers in sections or even single cells [3, 4].
Exfoliative oral cytology has an increasing role for the evaluation of oral potentially malignant lesions [4] and in recognising dysplasia in suspicious oral lesions with both high sensitivity and specificity [5, 6]. The use of auxiliary methods such as DNA image cytometry, AgNOR analysis and cell cycle immunohistochemistry can increase accuracy even further [5, 7]. A biopsy must be performed, however, whenever dysplasia is detected, not only because the architecture needs to be considered when grading lesions but also because invasion cannot be reliably assessed by exfoliative cytology alone. Dysplasia can be assessed cytologically using direct smears or by liquid-based cytology methods.
The recognised cytological features of oral dysplasia are:
- Nuclear hyperchromasia.
- Increased nuclear to cytoplasmic ratio.
- Anisonucleosis and nuclear pleomorphism.
- Irregularities of nuclear membrane.
- Nuclear crowding.
- Nuclear moulding, clumping and irregular distribution of chromatin.
Cytological diagnosis is skill dependent and the cytopathologist must be able to recognise regenerating oral epithelial cells, which often form aggregates of immature keratinocytes. These cells may exhibit an increased nuclear to cytoplasmic ratio and sometimes possess prominent nucleoli, but may show no other nuclear abnormalities. Radiation- and chemoradiation-induced changes including micronucleation must be considered when cytology is performed in the immediate post-irradiation setting [4, 8].
In the absence of accurate predictive biomarkers, histological examination of biopsies is the current standard for planning the management of oral potentially malignant lesions. A number of grading systems for evaluation of dysplasia have been proposed and are discussed in the next section. Unfortunately, a number of studies have demonstrated that agreement amongst even specialist oral and maxillofacial pathologists is only fair, with kappa values typically ranging between 0.5 and 0.6.
Binary grading systems that classify lesions into ‘low grade’ and ‘high grade’ show improved utility in terms of predictive value and inter-observer agreement [9]. An agreement level of around 80% was reached when pathologists were asked to discriminate between dysplasia (of any grade) and cases without dysplasia [10]. Group training relating to criteria for defining the features of dysplasia appears to increase reliability. Non-specialist pathologists tend to downgrade oral dysplasia compared to specialist oral and maxillofacial pathologists [9]. It is interesting that even when pathologists agree on the grade they may be basing their judgement on differing features [11].
Factors such as the presence or absence of lichenoid inflammation, clinical history (smoking and alcohol use) and lesion site have been reported to consistently influence dysplasia grading [12]. It is regrettable that clinical treatment planning has to be performed on the basis of pathology reports that show such poor reliability. Biomarkers are needed that have the power to predict biological behaviour but as yet no markers have proved to be sufficiently robust for clinical application. Clinical correlation can help, and close involvement of the pathologist with the clinical team is facilitated by specialist pathology reporting. Consensus reporting of oral dysplasia by a team of specialist pathologists also helps to improve reporting accuracy and over time develops greater consistency.
A dedicated laboratory team experienced in orientating mucosal specimens correctly and agreed protocols defining the number of levels prepared are also important in providing a high-quality service. Regular review (audit) and the monitoring of clinical outcomes in a comprehensive database that includes pathological findings are useful tools for developing optimal management protocols.
The relative rarity and huge variability of oral potentially malignant lesions makes it difficult to undertake randomised controlled trials. Prolonged follow-up times, which are needed for potential lesions to reach malignant transformation, also hamper such trials. The accrual of a biobank of samples of oral potentially malignant lesions accompanied by clinical data, including outcomes, is necessary for future biomarker evaluation. Input from specialist pathologists is therefore vital not only for the provision of day to day reporting services but also to the development of long-term goals.
Histopathological Features of Oral Potentially Malignant Disorders
The concept that squamous carcinoma has a prolonged preinvasive phase that offers the opportunity for therapeutic intervention was first recognised in the cervix. Indeed, early descriptions of the cytological and architectural histological features of oral precancerous lesions largely evolved from previous accounts of their cervical counterparts. Unfortunately, the epithelium of the uterine cervix is dissimilar in many ways to the oral mucosa, which is itself heterogeneous in nature, as described in Chapter 2.
The epithelium of the cervix is non-keratinising and varies during reproductive life. Cervical squamous epithelium differentiates by metaplasia from the reserve cell population that normally replenishes the columnar cells of the endocervical canal, and a transitional zone can be recognised histologically. Also in contrast to oral cancer, most cervical cancers are human papillomavirus (HPV) related and this may influence the morphology of cervical intraepithelial neoplasia (CIN) [13].
The CIN system encompasses six stages, from normal through reactive, CIN1, CIN2 and CIN3, to invasion; these can be mapped to the squamous intraepithelial neoplasia (SIN) system (Figure 6.2). These are thought to represent a serial progression but evidence for this is lacking due to the ethics constraints of designing a clinical trial to determine if there is a succession of events. In a widely quoted, extended and unethical clinical trial it was demonstrated that the 30-year cumulative risk of cervical cancer was 0.7% in women with />
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


