Bone
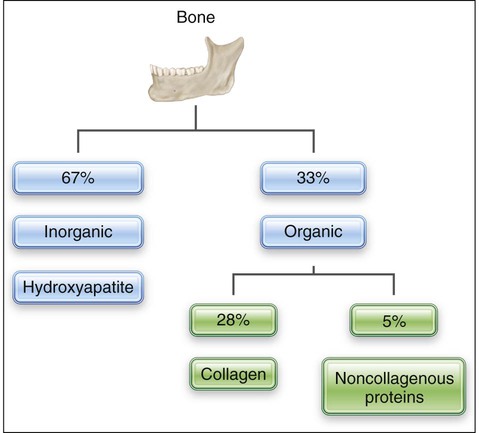
Bone is a mineralized connective tissue consisting by dry weight of about 28% type I collagen and 5% noncollagenous matrix proteins, such as bone sialoprotein, osteocalcin, osteonectin, osteopontin, and proteoglycans; growth factors and serum proteins also are found in bone (Table 6-1). This organic matrix is permeated by substituted hydroxyapatite (Ca10[PO4]6[OH]2), which makes up the remaining 67% of bone (Figure 6-1). The mineral is in the form of small plates, most of which lodge in the holes and pores of collagen fibrils (see Chapter 1).
TABLE 6-1
| APPEARANCE | BONE TYPE | EXAMPLE |
| Gross appearance | Flat | Skull, pelvis, scapula |
| Long | Appendicular skeleton | |
| Macroscopic appearance | Compact | Mature bone; flat bones and shaft of long bones |
| Spongy/cancellous/trabecular | Early embryonic bone; interior of extremities of long bones | |
| Development/formation | Intramembranous | Direct transformation of mesenchyme |
| Endochondral | From a cartilage model | |
| Regions | Diaphysis | Shaft |
| Metaphysis | Transitional portion of the shaft leading to the growth plate zone | |
| Epiphysis | Extremities of long bones | |
| Microstructure | Embryonic/woven | Irregular collagen network |
| Lamellar | Collagen arranged in concentric layers | |
| Disposition of lamellae | Circumferential | Found on periosteal and endosteal surfaces |
| Osteonic | Concentric lamellae forming osteons | |
| Interstitial | Residual fragments between osteons | |
| Types of osteons | Primary | The first formed haversian systems (osteons) consisting of poorly organized lamellae |
| Definitive | Higher orders of osteons formed after remodeling of primary osteons |
Gross Bone Histology
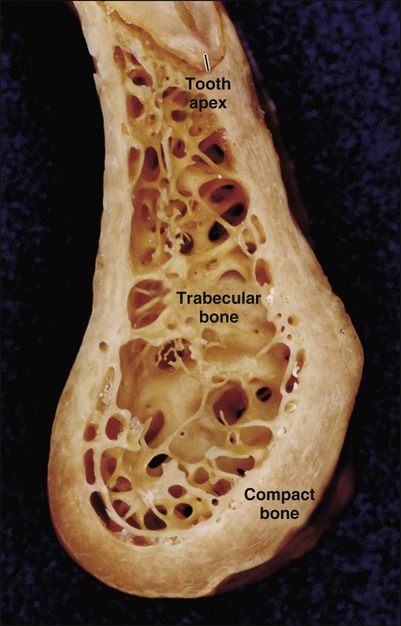
Characteristic of all bones are a dense outer sheet of compact bone and a central, medullary cavity. This cavity is filled with red or yellow bone marrow that is interrupted, particularly at the extremities of long bones, by a network of bone trabeculae (trabecular, cancellous, or spongy bone are the terms used to describe this network; Figure 6-2). These two types of bone behave differently and have different metabolic responses.
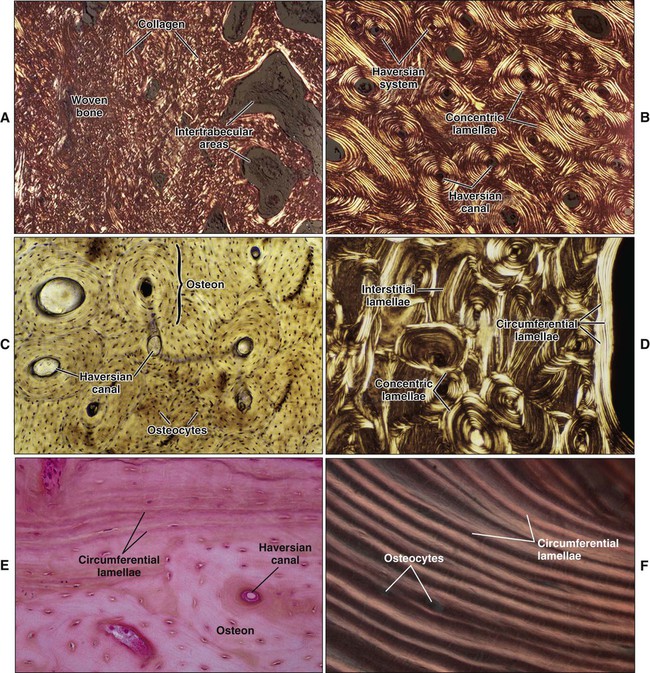
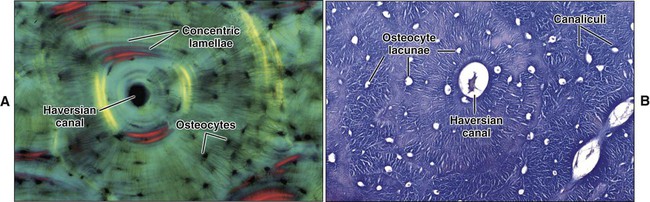
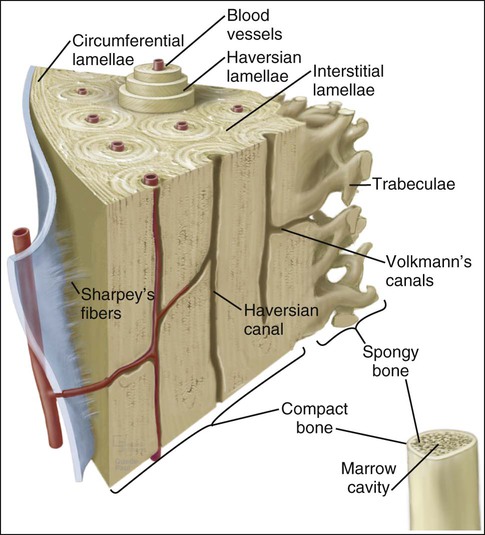
Mature or adult bones, whether compact or trabecular, are histologically identical in that they consist of microscopic layers or lamellae. Three distinct types of layering are recognized: circumferential, concentric, and interstitial (Figures 6-3 to 6-5). Circumferential lamellae enclose the entire adult bone, forming its outer and inner perimeters. Concentric lamellae make up the bulk of compact bone and form the basic metabolic unit of bone, the osteon (also called the haversian system) (see Figure 6-4). The osteon is a cylinder of bone, generally oriented parallel to the long axis of the bone. In the center of each is a canal, the haversian canal, which is lined by a single layer of bone cells that cover the bone surface; each canal houses a capillary. Adjacent haversian canals are interconnected by Volkmann canals; these channels, like haversian canals, contain blood vessels, thus creating a rich vascular network throughout compact bone. Interstitial lamellae are interspersed between adjacent concentric lamellae and fill the spaces between them. Interstitial lamellae are actually fragments of preexisting concentric lamellae from osteons created during remodeling that can take a multitude of shapes.
Surrounding the outer aspect of every compact bone is connective tissue membrane, the periosteum, which has two layers. The outer layer of the periosteum consists of a dense, irregular connective tissue termed the fibrous layer (see Figure 6-5). The inner layer of the periosteum, next to the bone surface, consists of bone cells, their precursors, and a rich microvascular supply. The internal surfaces of compact and cancellous bone are covered by endosteum. However, this layer is not well demarcated and consists of loose connective tissue containing osteogenic cells, that physically separates the bone surface from the marrow within. In general, the periosteal surface of bone is more active in bone formation than the endosteal one.
Refer to Table 6-1 for the descriptors used to describe the physical characteristics of bone.
Bone Cells
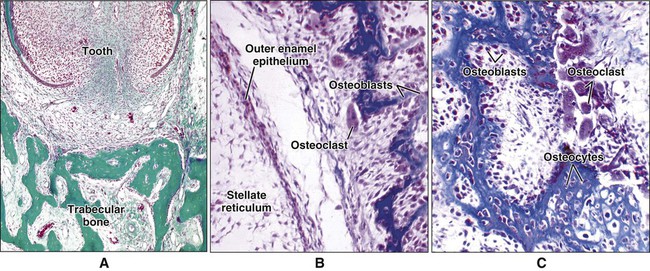
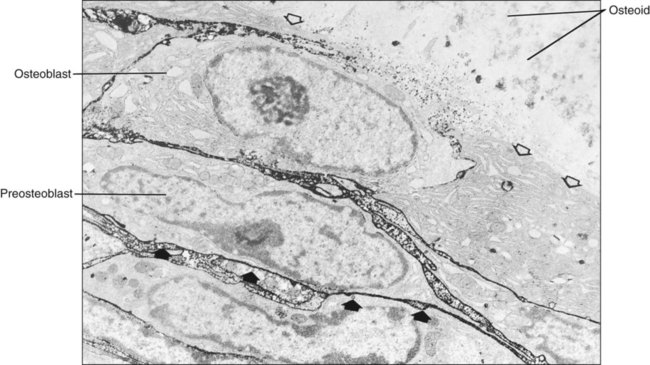
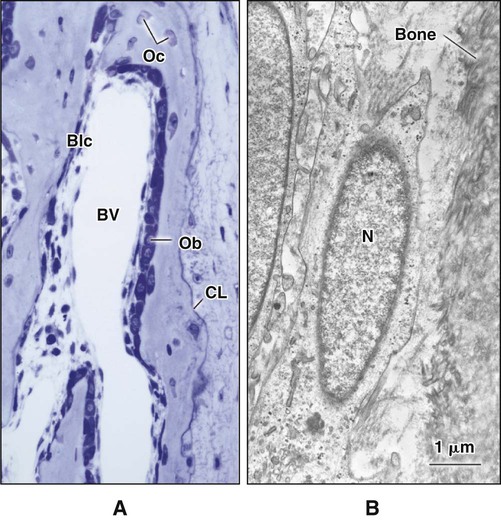
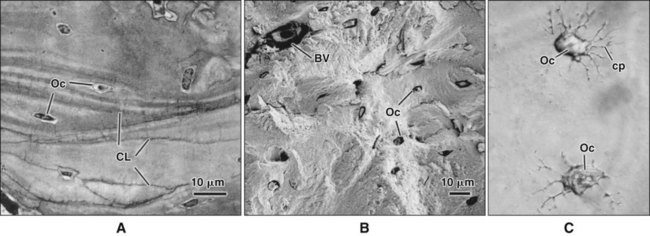
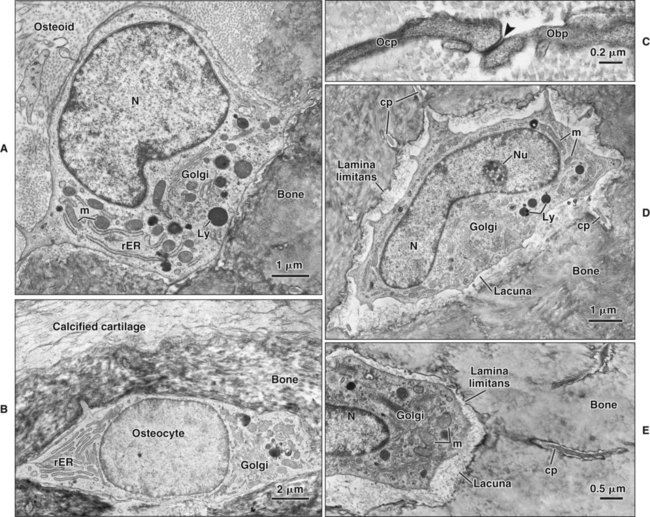
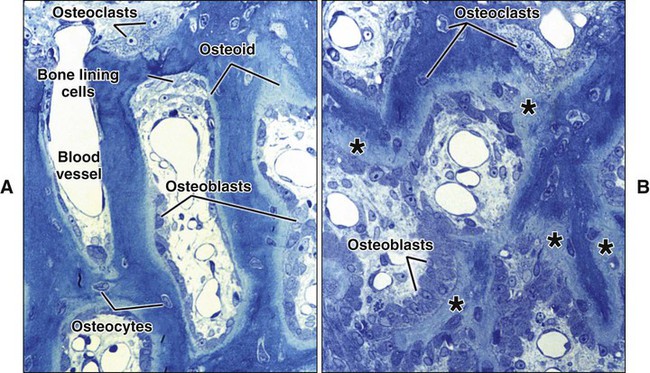
Different cells are responsible for the formation, resorption, and maintenance of osteoarchitecture. Two cell lineages are present in bone, each with specific functions: (1) osteogenic cells, which form and maintain bone, and (2) osteoclasts, which resorb bone (Figures 6-6 to 6-8). Osteogenic cells have variable morphology (including osteoprogenitors, preosteoblasts, osteoblasts, osteocytes, and bone lining cells) representing different maturational stages. The differentiation sequence from osteoprogenitor to preosteoblast does not show any distinctive morphologic features, and much research interest is focused on finding molecular markers for the various stages of the osteogenic life cycle.
Osteoblasts
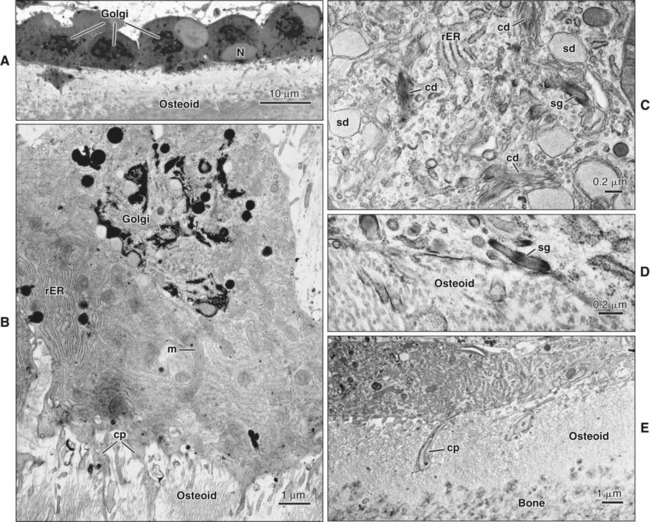
Osteoblasts are mononucleated cells that synthesize the organic matrix of bone. Osteoblasts arise from pluripotent stem cells, which are of mesenchymal origin in the axial and appendicular skeleton and of ectomesenchymal origin (neural crest cells that migrate in mesenchyme) in the head. Although osteoblasts are differentiated cells, both preosteoblasts and osteoblasts can undergo mitosis during prenatal development and occasionally during postnatal growth. Both cell types exhibit high levels of alkaline phosphatase activity on the outer surface of their plasma membrane (Figure 6-9). Functionally, the enzyme is believed to cleave inorganically bound phosphate. The liberated phosphate likely contributes to the initiation and progressive growth of bone mineral crystals. However, the function of alkaline phosphatase in bone-forming cells is likely complex and is not yet defined completely. Recently, it has been shown that “active” osteoblasts express a membrane protein called Bril, a member of the interferon inducible transmembrane protein family (Ifitm) (Figure 6-10). Like alkaline phosphatase, the precise function of Bril is not yet fully defined, but this protein is a marker of sites where bone is actively forming.
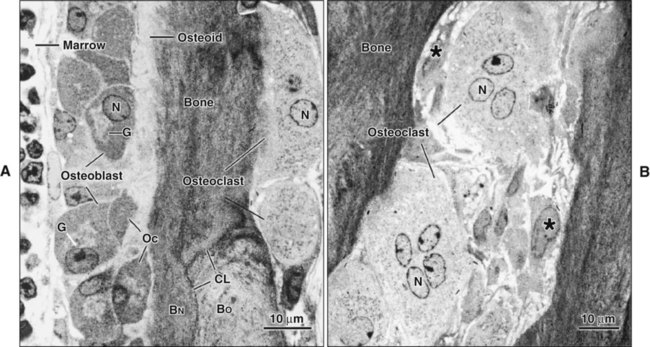
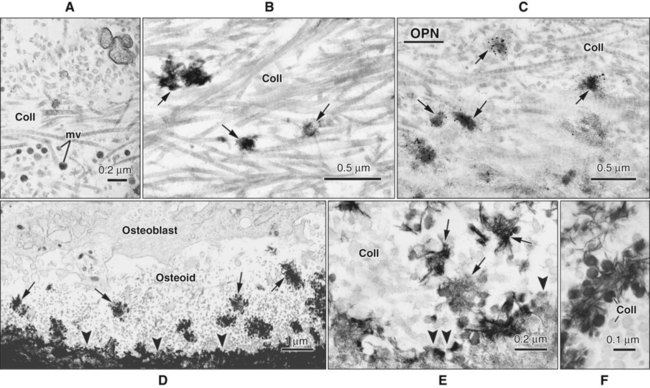
Osteoblasts are plump, cuboidal cells (when very active) or slightly flattened cells that are primarily responsible for the production of the organic matrix of bone (Figure 6-11; see also Figures 6-7 and 6-8). They exhibit abundant and well-developed protein synthetic organelles. At the light microscopic level, the Golgi complex characteristically appears as a clear, paranuclear area that can be defined easily following cytochemical reactions for Golgi-resident enzymes (see Figures 6-8, A, and 6-11, A and B). The secretory products of osteoblasts include type I collagen, the dominant component of the organic matrix, small amounts of type V collagen and proteoglycans, and several noncollagenous proteins. The collagen type I molecule is formed and assembled, as in fibroblasts and odontoblasts (see Chapters 4 and 8), within the rough endoplasmic reticulum and Golgi compartments. The spherical and cylindrical distentions of the Golgi complex are believed to represent different stages of procollagen assembly (Figure 6-11, C). The typical elongated, electron-dense, collagen-containing secretory granules release their contents primarily along the surface of the cell apposed to forming bone. These molecules assemble extracellularly as fibrils and accumulate as a layer of uncalcified matrix called osteoid (prebone) (Figure 6-11, D and E). Some debate still continues as to whether the noncollagenous proteins are contained within the collagen secretory granules or in a distinct population of granules. Irrespective of this aspect, noncollagenous proteins also are released mainly along the surface of osteoblasts apposed to osteoid and diffuse from the osteoblast surface toward the mineralization front where they participate in regulating mineral deposition. Near the mineralization front, mineralization foci can be seen within osteoid, and certain noncollagenous proteins, such as bone sialoprotein and osteopontin, accumulate within them (Figure 6-12).
In addition to structural matrix proteins, osteoblasts, their precursors, or both secrete a number of cytokines and growth factors that help regulate cellular function and bone formation. These include several members of the bone morphogenetic protein (BMP) superfamily such as BMP-2, BMP-7, and transforming growth factor β, in addition to the insulin-like growth factors (IGF-I and IGF-II), platelet-derived growth factor, and fibroblastic growth factor. Although the timing of secretion and the complex interactions of these growth factors remain to be clarified, the combinations of IGF-I, transforming growth factor β, and platelet-derived growth factor increase the rapidity of bone formation and bone repair and are being considered for dental therapy. For instance, these combinations may be used to speed healing and bone growth after periodontal surgery or to prevent periodontal disease by the early treatment of periodontal pockets (see Chapter 15). Similarly, these factors may be used to enhance osseous integration after placement of dental implants.
The hormones most important in bone metabolism are parathyroid hormone (PTH), 1,25-dihydroxyvitamin D, calcitonin, estrogen, and the glucocorticoids. The actions of parathyroid hormone and vitamin D are dual, enhancing bone resorption at high (pharmacologic) concentrations but supporting bone formation at lower (physiologic) concentrations. Calcitonin and estrogen inhibit resorption, whereas the glucocorticoids inhibit resorption and formation (but primarily formation). The hormones affecting bone most likely work primarily through altering the secretion of cytokines and growth factors. Evidence is increasing that centrally mediated mechanisms also are involved in bone metabolism. Leptin, a circulating hormone produced by adipocytes, inhibits the release of brainstem-derived serotonin, which favors bone mass accrual and appetite through its action on hypothalamic neurons. This hormone acts on the hypothalamus and, through involvement of the sympathetic nervous system, can promote and inhibit the differentiation of osteoclasts. Some evidence also indicates that leptin also may work locally to promote the differentiation of osteoprogenitor cells and stimulate osteoblasts to make new bone. Osteoblasts form a cell layer over the forming bone surface and have been proposed to act as a barrier to control ion flux into and out of bone. Although there are no junctional complexes between cells, gap junctions do form and functionally couple adjacent cells. When bone is no longer forming, osteoblasts flatten substantially, extending along the bone surface (Figure 6-13; see also Figure 6-7, A). These cells, termed bone lining cells, contain few synthetic organelles, suggesting that they are less implicated in the production of matrix proteins. Bone lining cells cover most surfaces in the adult skeleton. It has been postulated that bone lining cells retain their gap junctions with osteocytes, creating a network that functions to control mineral homeostasis and ensure bone vitality. Such quiescent bone surfaces are believed to be the primary site for mineral exchange between blood and bone.
Osteocytes
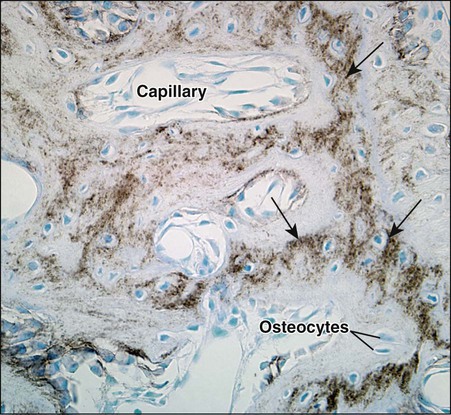
As osteoblasts form bone, some become trapped in the matrix they secrete, whether mineralized or unmineralized; these cells then are called osteocytes (Figures 6-14 and 6-15; see also Figures 6-4, 6-6, and 6-7). The number of osteoblasts that become osteocytes varies depending on the rapidity of bone formation; the more rapid the formation, the more osteocytes are present per unit volume. As a general rule, embryonic (woven) bone and repair bone have more osteocytes than does lamellar bone (Figure 6-16; see also Figure 6-6, C).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses