Salivary Glands
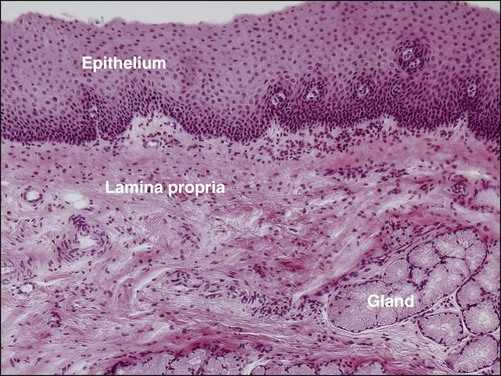
In human beings, three pairs of major salivary glands—the parotid, submandibular, and sublingual—are located outside the oral cavity, with extended duct systems through which the gland secretions reach the mouth. Numerous smaller minor salivary glands are located in various parts of the oral cavity—the labial, lingual, palatal, buccal, glossopalatine, and retromolar glands—typically located in the submucosal layer (Figure 11-1), with short ducts opening directly onto the mucosal surface.
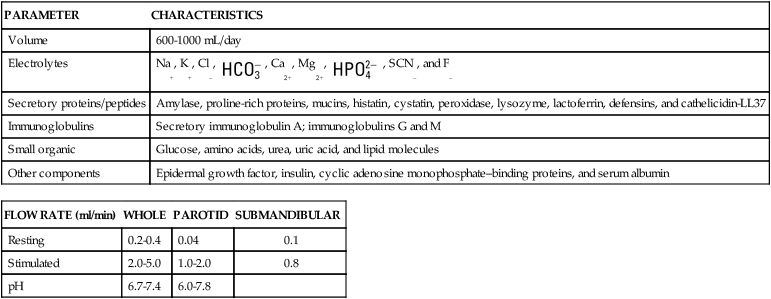
The composition of saliva is summarized in Table 11-1. The saliva produced by each major salivary gland, however, differs in amount and composition. The parotid glands secrete a watery saliva rich in enzymes such as amylase, proteins such as the proline-rich proteins, and glycoproteins. Submandibular saliva, in addition to the components already listed, contains highly glycosylated substances called mucins. The sublingual gland produces viscous saliva also rich in mucins. Oral fluid, which is referred to as mixed, or whole, saliva, includes the secretions of the major glands, the minor glands, desquamated oral epithelial cells, microorganisms and their products, food debris, and serum components and inflammatory cells that gain access through the gingival crevice. Moreover, whole saliva is not the simple sum of all of these components because many of the proteins are removed as they adhere to the surfaces of the teeth and oral mucosa, bind to microorganisms, or are degraded.
TABLE 11-1
| PARAMETER | CHARACTERISTICS |
| Volume | 600-1000 mL/day |
| Electrolytes | Na+, K+, Cl–, 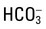 , Ca2+, Mg2+, , Ca2+, Mg2+, 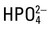 , SCN–, and F– , SCN–, and F– |
| Secretory proteins/peptides | Amylase, proline-rich proteins, mucins, histatin, cystatin, peroxidase, lysozyme, lactoferrin, defensins, and cathelicidin-LL37 |
| Immunoglobulins | Secretory immunoglobulin A; immunoglobulins G and M |
| Small organic | Glucose, amino acids, urea, uric acid, and lipid molecules |
| Other components | Epidermal growth factor, insulin, cyclic adenosine monophosphate–binding proteins, and serum albumin |
| FLOW RATE (ml/min) | WHOLE | PAROTID | SUBMANDIBULAR |
| Resting | 0.2-0.4 | 0.04 | 0.1 |
| Stimulated | 2.0-5.0 | 1.0-2.0 | 0.8 |
| pH | 6.7-7.4 | 6.0-7.8 |
Functions of Saliva
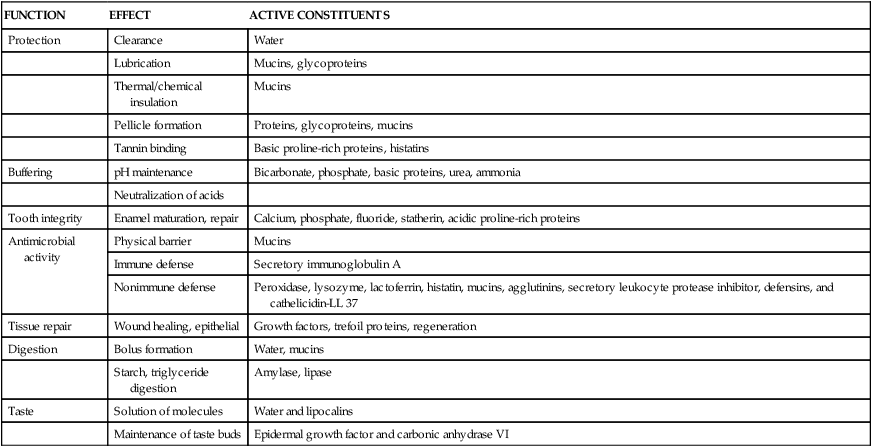
Saliva has many functions (Table 11-2), the most important being protection of the oral cavity.
TABLE 11-2
| FUNCTION | EFFECT | ACTIVE CONSTITUENTS |
| Protection | Clearance | Water |
| Lubrication | Mucins, glycoproteins | |
| Thermal/chemical insulation | Mucins | |
| Pellicle formation | Proteins, glycoproteins, mucins | |
| Tannin binding | Basic proline-rich proteins, histatins | |
| Buffering | pH maintenance | Bicarbonate, phosphate, basic proteins, urea, ammonia |
| Neutralization of acids | ||
| Tooth integrity | Enamel maturation, repair | Calcium, phosphate, fluoride, statherin, acidic proline-rich proteins |
| Antimicrobial activity | Physical barrier | Mucins |
| Immune defense | Secretory immunoglobulin A | |
| Nonimmune defense | Peroxidase, lysozyme, lactoferrin, histatin, mucins, agglutinins, secretory leukocyte protease inhibitor, defensins, and cathelicidin-LL 37 | |
| Tissue repair | Wound healing, epithelial | Growth factors, trefoil proteins, regeneration |
| Digestion | Bolus formation | Water, mucins |
| Starch, triglyceride digestion | Amylase, lipase | |
| Taste | Solution of molecules | Water and lipocalins |
| Maintenance of taste buds | Epidermal growth factor and carbonic anhydrase VI |
Anatomy
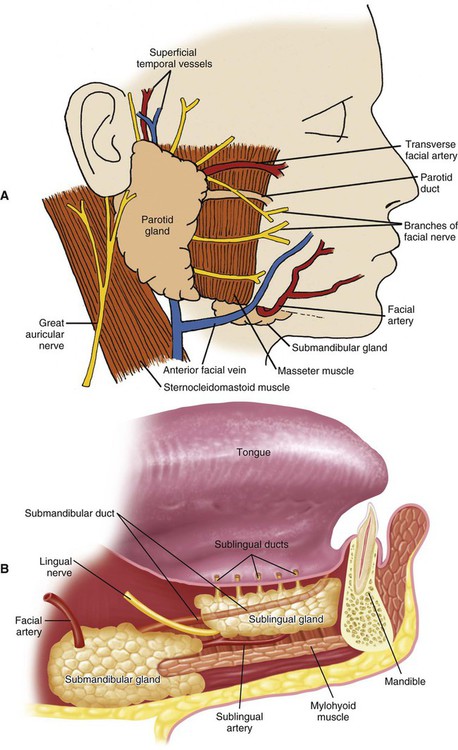
The parotid gland is the largest salivary gland. The superficial portion of the parotid gland is located subcutaneously, in front of the external ear, and its deeper portion lies behind the ramus of the mandible. The parotid gland is associated intimately with peripheral branches of the facial nerve (cranial nerve VII; Figure 11-2, A). The duct (Stensen’s duct) of the parotid gland runs forward across the masseter muscle, turns inward at the anterior border of the masseter, and opens into the oral cavity at a papilla opposite the maxillary second molar. A small amount of parotid tissue occasionally forms an accessory gland associated with Stensen’s duct, just anterior to the superficial portion. The parotid gland receives its blood supply from branches of the external carotid artery as they pass through the gland. The parasympathetic nerve supply to the parotid gland is mainly from the glossopharyngeal nerve (cranial nerve IX). The preganglionic fibers synapse in the otic ganglion; the postganglionic fibers reach the gland through the auriculotemporal nerve. The sympathetic innervation of all of the salivary glands is provided by postganglionic fibers from the superior cervical ganglion, traveling with the blood supply.
The submandibular gland is situated in the posterior part of the floor of the mouth, adjacent to the medial aspect of the mandible and wrapping around the posterior border of the mylohyoid muscle (Figure 11-2, B). The excretory duct (Wharton’s duct) of the submandibular gland runs forward above the mylohyoid muscle and opens into the mouth beneath the tongue at the sublingual caruncle, lateral to the lingual frenum. The submandibular gland receives its blood supply from the facial and lingual arteries. The parasympathetic nerve supply is derived mainly from the facial nerve (cranial nerve VII), reaching the gland through the lingual nerve and submandibular ganglion.
The sublingual gland is the smallest of the paired major salivary glands. The gland is located in the anterior part of the floor of the mouth between the mucosa and the mylohyoid muscle (see Figure 11-2, B). The secretions of the sublingual gland enter the oral cavity through a series of small ducts (ducts of Rivinus) opening along the sublingual fold and often through a larger duct (Bartholin’s duct) that opens with the submandibular duct at the sublingual caruncle. The sublingual gland receives its blood supply from the sublingual and submental arteries. The facial nerve (cranial nerve VII) provides the parasympathetic innervation of the sublingual gland, also via the lingual nerve and submandibular ganglion.
Development
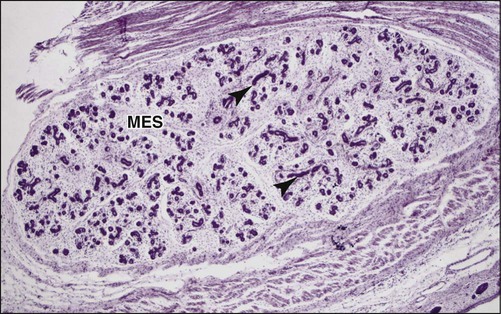
Just as for teeth, the individual salivary glands arise as a proliferation of oral epithelial cells, forming a focal thickening that grows into the underlying ectomesenchyme. Continued growth results in the formation of a small bud connected to the surface by a trailing cord of epithelial cells, with mesenchymal cells condensing around the bud (Figure 11-3). Clefts develop in the bud, forming two or more new buds; continuation of this process, called branching morphogenesis, produces successive generations of buds and a hierarchic ramification of the gland.
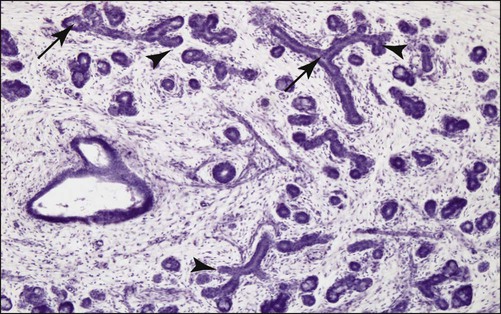
The development of a lumen within the branched epithelium generally occurs in this order: (1) in the distal end of the main cord and in branch cords, (2) in the proximal end of the main cord, and (3) in the central portion of the main cord (Figure 11-4). The lumina form within the ducts before they develop within the terminal buds. Some studies have suggested that lumen formation may involve apoptosis of centrally located cells in the cell cords, but further research is required to establish definitively a role for cell death in this process.
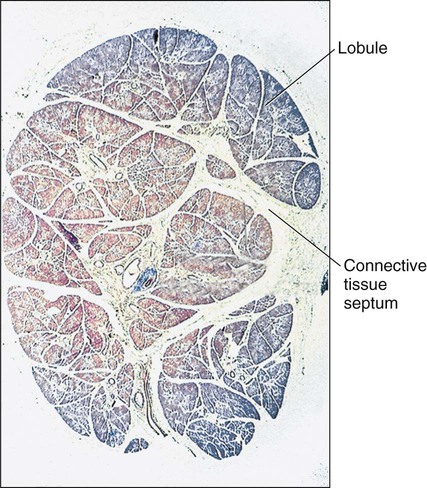
Following development of the lumen in the terminal buds, the epithelium consists of two layers of cells. The cells of the inner layer eventually differentiate into the secretory cells of the mature gland, mucous or serous, depending on the specific gland. Some cells of the outer layer form the contractile myoepithelial cells that are present around the secretory end pieces and intercalated ducts. As the epithelial parenchymal components increase in size and number, the associated mesenchyme (connective tissue) is diminished, although a thin layer of connective tissue remains, surrounding each secretory end piece and duct of the adult gland. Thicker partitions of connective tissue (septa), continuous with the capsule and within which run the nerves and blood vessels supplying the gland, invest the excretory ducts and divide the gland into lobes and lobules (Figure 11-5).
Structure
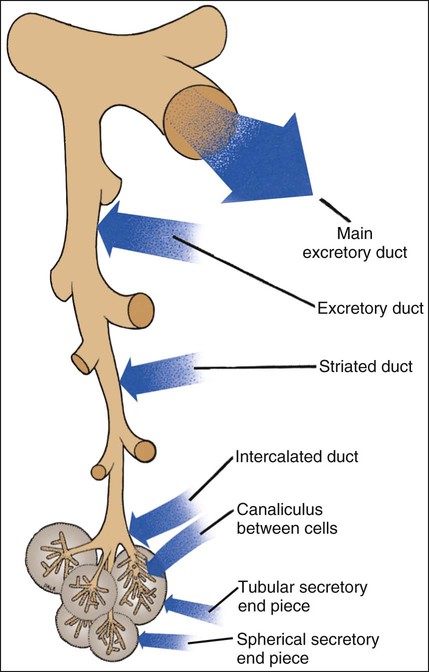
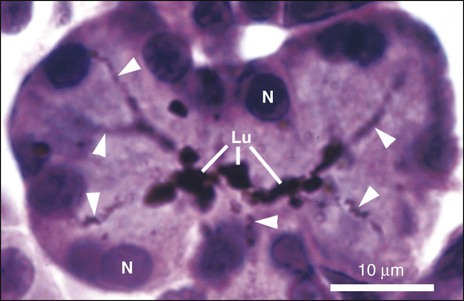
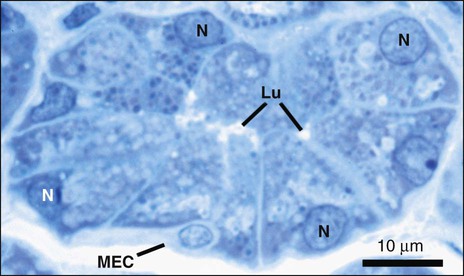
As described in the previous section, a salivary gland consists of a series of branched ducts, terminating in spherical or tubular secretory end pieces or acini (Figure 11-6). An analogy can be made to a bunch of grapes, with the stems representing the ducts and the grapes corresponding to the secretory end pieces. The main excretory duct, which empties into the oral cavity, divides into progressively smaller interlobar and interlobular excretory ducts that enter the lobes and lobules of the gland. The predominant intralobular ductal component is the striated duct, which plays a major role in modification of the primary saliva produced by the secretory end pieces. Connecting the striated ducts to the secretory end pieces are intercalated ducts, which branch once or twice before joining individual end pieces. The lumen of the end piece is continuous with that of the intercalated duct. In some glands, small extensions of the lumen, intercellular canaliculi, are found between adjacent secretory cells (Figure 11-7). These intercellular canaliculi may extend almost to the base of the secretory cells and serve to increase the size of the secretory (luminal) surface of the cells.
Secretory Cells
Serous Cells
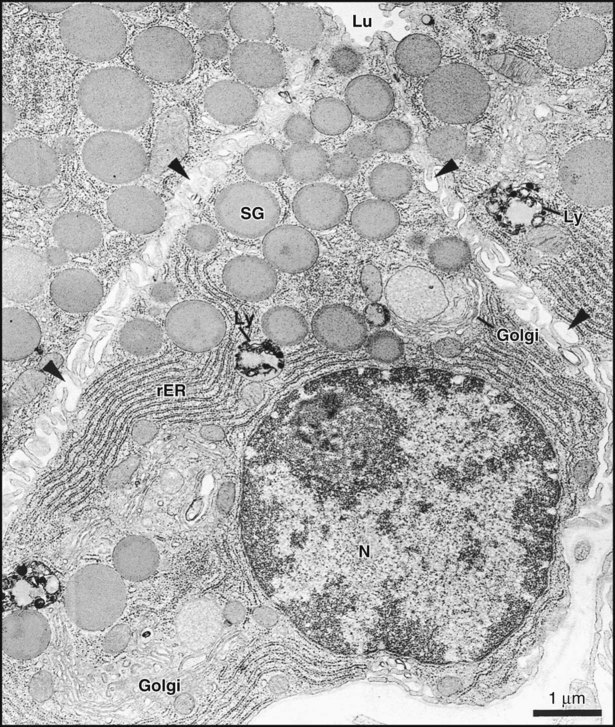
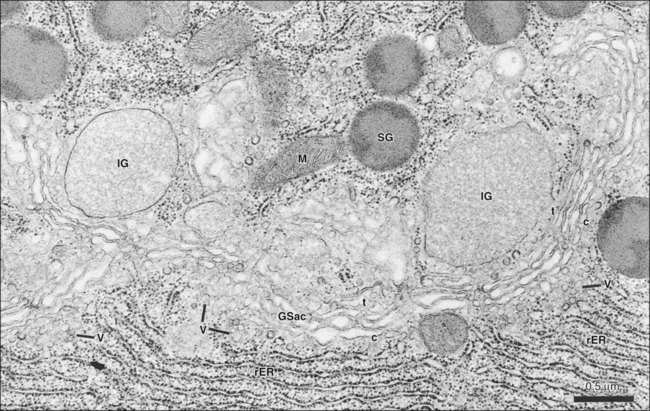
Secretory end pieces that are composed of serous cells are typically spherical and consist of 8 to 12 cells surrounding a central lumen (Figure 11-8). The cells are pyramidal, with a broad base adjacent to the connective tissue stroma and a narrow apex forming part of the lumen of the end piece. The lumen usually has fingerlike extensions located between adjacent cells called intercellular canaliculi that increase the size of the luminal surface of the cells. The spherical nuclei are located basally, and occasionally, binucleated cells are seen. Numerous secretory granules, in which the macromolecular components of saliva are stored, are present in the apical cytoplasm (Figures 11-9 and 11-10). The granules may have a variable appearance, ranging from homogeneously electron-dense to a combination of electron-dense and electron-lucent regions arranged in intricate patterns. The basal cytoplasm contains numerous cisternae of rough endoplasmic reticulum, which converge on a large Golgi complex located just apical or lateral to the nucleus (Figure 11-11). Forming secretory granules of variable size and density are present at the trans face of the Golgi complex. These granules increase in density as their content condenses, eventually forming the mature secretory granules. Serous cells also contain all of the typical organelles found in other cells, including cytoskeletal components, mitochondria, lysosomes, and peroxisomes.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses