Embryology of the Head, Face, and Oral Cavity
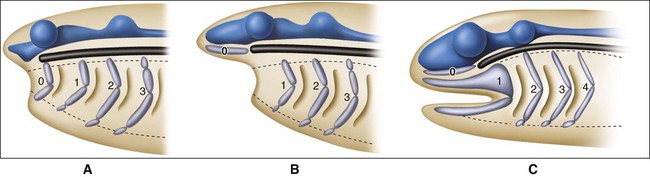
Knowledge of the evolutionary development of the skull, face, and jaws is helpful in understanding the complex events involved in cephalogenesis (formation of the head). Early chordates have a fairly simple anatomic plan with (1) a notochord for support, (2) a simple nervous system and sense organs, (2) segmented muscle blocks, and, at the beginning of the pharynx in its lateral wall, (3) a series of branchial clefts supported by cartilage to permit gaseous exchange. The first vertebrates evolved from this simple plan and were jawless (agnathia). Cartilaginous blocks (occipital and parachordal) evolved to support the notochord in the head region, along with cartilaginous capsules (nasal, optic, and otic) to protect the sense organs. These cartilages collectively form the neurocranium. The branchial arches, as mentioned, are supported by a series of cartilaginous rods originally numbered 0, 1, 2, and so on, that constitute the viscerocranium. The first cartilage (cartilage 0) of the branchial arches migrated to the neurocranium to provide additional support as the trabecular cartilage. Because of this, the actual second arch cartilage became the first arch cartilage (Figure 3-1, A and B). The neurocranium and viscerocranium together form the chondrocranium.
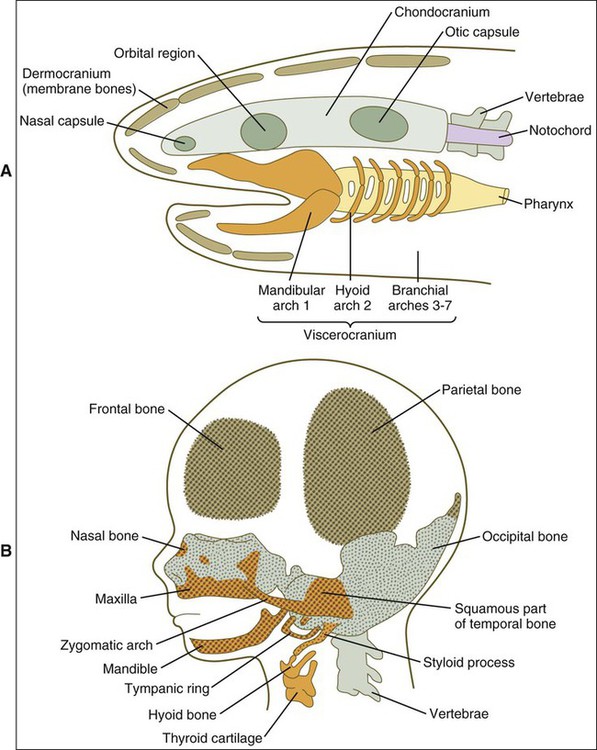
From this simple model, vertebrates came to possess jaws (gnathostomata) through modification of the jointed first arch cartilage, with the upper element, the palatopterygo quadrate bar, becoming the upper jaw and the lower element, Meckel’s cartilage, becoming the lower jaw (Figure 3-1, C). The fibrous connection between the two formed the jaw joint. In addition to jaws, vertebrate evolution also brought about massive expansion of the head region and associated larger neural and sensory elements. For protection, dermal bones developed as additional bony skeletal elements to form the vault of the skull and the facial skeleton, which included bony jaws and teeth. This cephalic expansion demanded a source of new connective tissue, and as explained in Chapter 2, this source is the neuroectoderm, from which neural crest cells (NCCs) migrate and differentiate into ectomesenchyme. Figure 3-2 shows a comparison between the cranial components of the primitive vertebrate skull and the cranial skeleton of a human fetus.
Neural Crest Cells and Head Formation
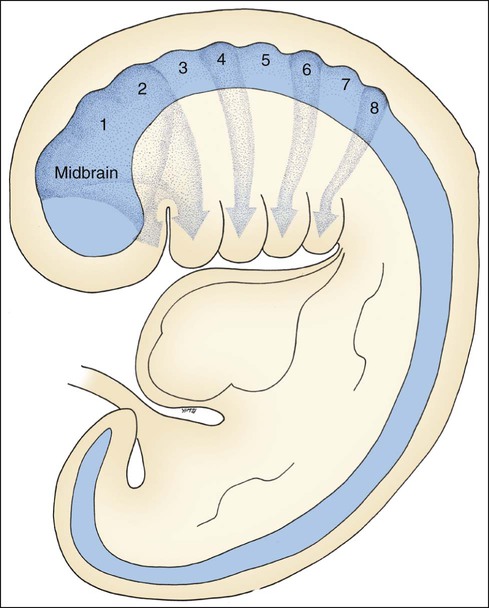
The folding of the three-layered embryo has been described, and the rostral or head fold is important at this point. The neural tube is produced by the formation and fusion of the neural folds, which sink beneath surface ectoderm. The anterior portion of this neural tube expands greatly as the forebrain, midbrain, and hindbrain form, and the part associated with the hindbrain develops a series of eight bulges, the rhombomeres (Figure 3-3). Lateral to the neural tube is paraxial mesoderm, which partially segments rostrally to form seven somatomeres and fully segments caudally to form somites, the first in the series being the occipital somites.
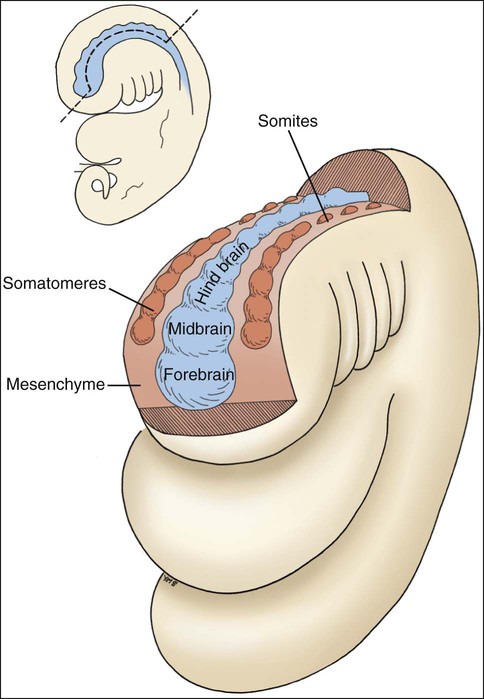
NCCs from the midbrain and the first two rhombomeres transform and migrate as two streams to supply additional embryonic connective tissue needed for craniofacial development (Figure 3-4). The first stream provides much of the ectomesenchyme associated with the face while the second stream is targeted to the first arch where they contribute to formation of the jaws. NCC subpopulations, depending on their anteroposterior location along the neural tube, are subject to a very complex temporal and spatial set of signaling event. A plethora of molecules are used as cues to guide them to their ultimate destination within restricted areas of the head. Their eventual differentiation is also tightly controlled through reciprocal signaling with neighboring ectodermal cells. The various intracellular signaling events and crosstalk between cells eventually culminate to elicit various cellular responses including proliferation, migration, differentiation, and survival or apoptosis.
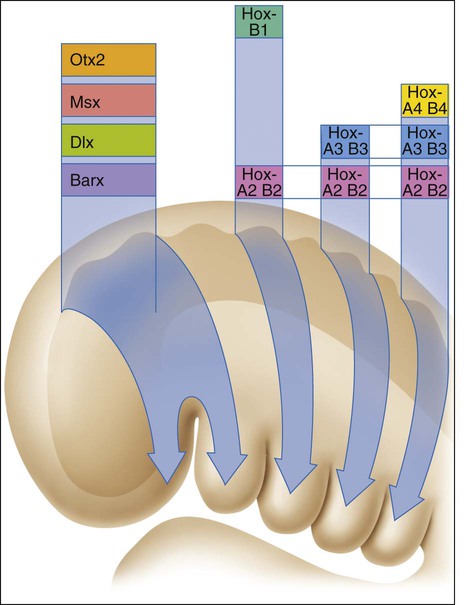
NCCs from rhombomere 3 and beyond migrate into arches that will give rise to pharyngeal structures. Because homeobox transcription factor genes are not expressed anterior to rhombomere 3, a different set of coded patterning genes has been adapted for development of cephalic structures (Figure 3-5). This new set of transcription factor genes, reflecting the later development of the head in evolutionary terms, includes orthodenticle homeobox 2 (Otx2), muscle segment homeobox (Msx), the distal-less homeobox (Dlx), and the BarH-like homeobox (Barx). Homeobox genes also are implicated in dental development, and their effects are discussed in Chapter 5.
Branchial (Pharyngeal) Arches and the Primitive Mouth
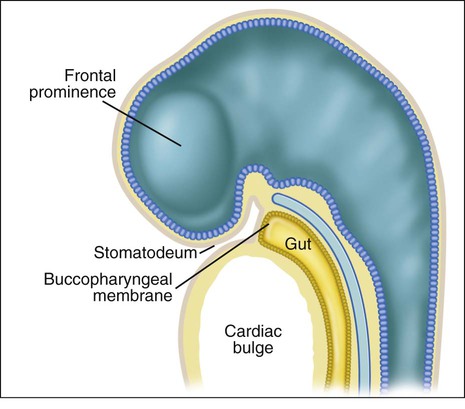
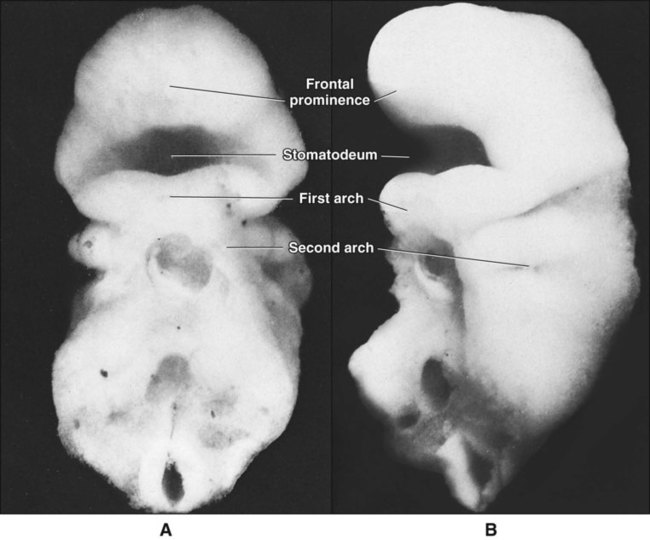
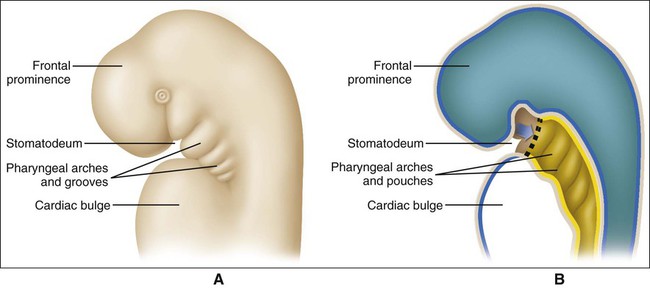
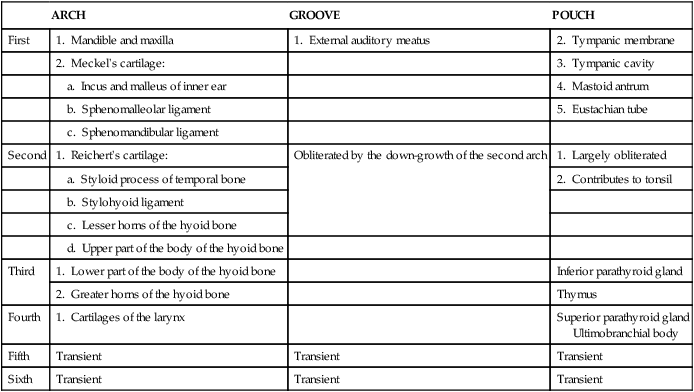
When the stomatodeum first forms, it is delimited rostrally by the frontal prominence and caudally by the developing cardiac bulge (Figures 3-6 and 3-7). The buccopharyngeal membrane, a bilaminar structure consisting of apposed ectoderm and endoderm, separates the stomatodeum from the foregut, but this soon breaks down so that the stomatodeum communicates directly with the foregut (see Figures 3-6 and 3-7). Laterally the stomatodeum becomes limited by the first pair of pharyngeal or branchial arches (Figure 3-8). The branchial arches form in the pharyngeal wall as a proliferation of mesoderm infiltrated by migrating NCCs. Six cylindrical thickenings thus form, however the fifth and sixth are transient structures in humans. They expand from the lateral wall of the pharynx and approach their anatomic counterparts expanding from the opposite side. In doing so, the arches progressively separate the primitive stomatodeum from the developing heart. The arches are seen clearly as bulges on the lateral aspect of the embryo and are separated externally by small clefts called branchial grooves. On the inner aspect of the pharyngeal wall are corresponding small depressions called pharyngeal pouches that separate each of the branchial arches internally. Table 3-1 summarizes the derivatives of the branchial (pharyngeal) arch system.
TABLE 3-1
Derivatives of the Branchial (Pharyngeal) Arch System
| ARCH | GROOVE | POUCH | |
| First | 1. Mandible and maxilla | 1. External auditory meatus | 2. Tympanic membrane |
| 2. Meckel’s cartilage: | 3. Tympanic cavity | ||
| a. Incus and malleus of inner ear | 4. Mastoid antrum | ||
| b. Sphenomalleolar ligament | 5. Eustachian tube | ||
| c. Sphenomandibular ligament | |||
| Second | 1. Reichert’s cartilage: | Obliterated by the down-growth of the second arch | 1. Largely obliterated |
| a. Styloid process of temporal bone | 2. Contributes to tonsil | ||
| b. Stylohyoid ligament | |||
| c. Lesser horns of the hyoid bone | |||
| d. Upper part of the body of the hyoid bone | |||
| Third | 1. Lower part of the body of the hyoid bone | Inferior parathyroid gland | |
| 2. Greater horns of the hyoid bone | Thymus | ||
| Fourth | 1. Cartilages of the larynx | Superior parathyroid gland Ultimobranchial body |
|
| Fifth | Transient | Transient | Transient |
| Sixth | Transient | Transient | Transient |
Anatomy of an Arch
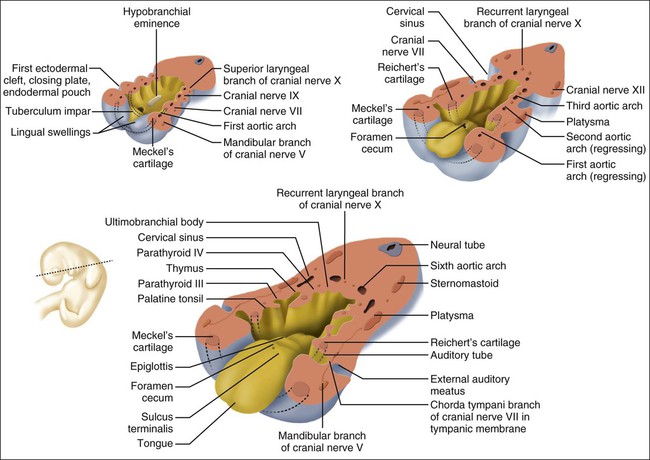
Every branchial arch has the same basic plan. The inner aspect is covered by endoderm and the outer surface by ectoderm, except for the first arch because it forms in front of the buccopharyngeal membrane and therefore derives completely from ectodermally covered surfaces. The central core consists of mesenchyme derived from lateral plate mesoderm invaded by NCCs, referred to as ectomesenchyme. This “neural-derived” mesenchyme condenses to form a bar of cartilage, the arch cartilage (Figure 3-9). The cartilage of the first arch is called Meckel’s cartilage, and that of the second Reichert’s, after the anatomists who first described them. The other arch cartilages are not named. The contribution of Meckel’s is discussed subsequently and Reichert’s cartilage gives rise to a bony process, the stylohyoid ligament and the upper part of the body and lesser horns of the hyoid bone. The cartilage of the third arch gives rise to the lower part of the body and greater horns of the hyoid bone and that of the fourth arch to the cartilages of the larynx.
Some of the mesenchyme surrounding this cartilaginous bar develops into striated muscle. The first arch musculature gives origin to the muscles of mastication, and the second arch musculature to the muscles of facial expression. Each arch also contains an artery and a nerve (Table 3-2). The nerve consists of two components, one motor (supplying the muscle of the arch) and one sensory. The sensory nerve divides into two branches: a posttrematic branch, supplying the epithelium that covers the anterior half of the arch, and a pretrematic branch, passing forward to supply the epithelium that covers the posterior half of the preceding arch. The nerve of the first arch is the fifth cranial (or trigeminal) nerve, that of the second is the seventh cranial (or facial) nerve, and that of the third is the ninth cranial (or glossopharyngeal) nerve. Structures derived from any arch carry with them the nerve supply of that arch. Thus the muscles of mastication are innervated by the trigeminal nerve.
TABLE 3-2
Innervation and Vascularization of Pharyngeal Arches
| ARCH | BLOOD VESSEL | NERVE |
| First | First aortic arch | Mandibular (and maxillary) division of the trigeminal nerve (cranial nerve V) |
| Second | Second aortic arch | Facial (VII) |
| Third | Third aortic arch | Glossopharyngeal (IX) |
| Fourth | Fourth aortic arch | Vagus (X) |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses