Periodontium
The periodontium is defined as those tissues supporting and investing the tooth and consists of cementum, periodontal ligament (PDL), bone lining the alveolus (socket), and that part of the gingiva facing the tooth. Proper functioning of the periodontium is achieved only through structural integrity and interaction between these various tissues. Together, these tissues form a specialized fibrous joint, a gomphosis, the components of which are of ectomesenchymal origin. The widespread occurrence of periodontal diseases and the realization that periodontal tissues lost to disease can be repaired has resulted in considerable effort to understand the factors and cells regulating the formation, maintenance, and regeneration of the periodontium. This chapter describes the histologic events leading to the formation of supporting tissues (Figure 9-1), except for the dentogingival junction, which is covered under oral mucosa (see Chapter 12).
Cementum
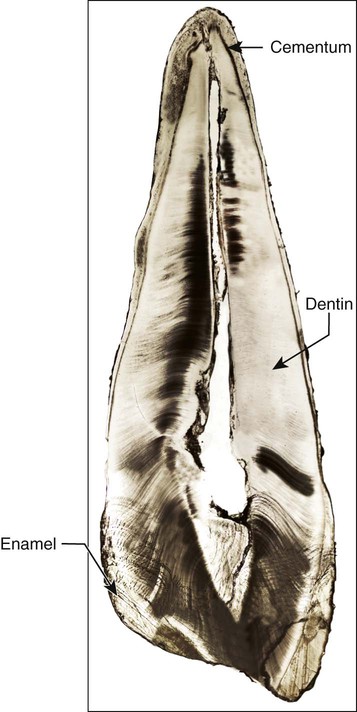
Cementum is a hard, avascular connective tissue that covers the roots of teeth (Figure 9-2). Cementum is classified according to the presence or absence of cells within its matrix and the origin of the collagen fibers of the matrix. The development of cementum has been subdivided into a prefunctional stage, which occurs throughout root formation, and a functional stage, which starts when the tooth is in occlusion and continues throughout life. Several varieties of cementum exist. The beginning student, however, needs only to think of the two main forms of cementum that have different structural and functional characteristics: acellular cementum, which provides attachment for the tooth, and cellular cementum, which has an adaptive role in response to tooth wear and movement and is associated with repair of periodontal tissues.
Biochemical Composition
Four mineralized tissues are found in the oral cavity, and three of these—enamel, dentin, and cementum—are components of the tooth. Their characteristics and biochemical composition are summarized in Table 1-1. The composition of cementum is similar to that of bone. Cementum is approximately 45% to 50% hydroxyapatite by weight and the remaining portion is collagen and noncollagenous matrix proteins. Type I collagen is the predominant collagen of cementum (constitutes up to 90% of the organic components in cellular cementum); in cellular intrinsic fiber cementum, just as in bone, it accommodates mineral deposition. Type I collagen is also the major collagen within the PDL region, and its main function is to structure the fiber bundles that anchor the tooth to the bone and distribute masticatory forces. Other collagens associated with cementum include type III, a less cross-linked collagen found in high concentrations during development and during repair and regeneration of mineralized tissues but is reduced with maturation of this tissue, and type XII collagen, a fibril-associated collagen with interrupted triple helices that binds to type I collagen and to noncollagenous proteins. Type XII collagen is found in high concentrations in ligamentous tissues, including the PDL, with lower levels noted in cementum. This nonfibrillar collagen interacts with type I collagen and may assist in maintaining a functional and mature PDL that can withstand the forces of occlusion. Trace amounts of other collagens, including types V, VI, and XIV, also are found in extracts of mature cementum; however, these may be contaminants from the PDL region, produced by PDL fibroblasts associated with collagen fibers inserted into cementum. Noncollagenous proteins identified in cementum also are associated with bone and include the following: alkaline phosphatase, bone sialoprotein, dentin matrix protein 1, dentin sialoprotein, fibronectin, osteocalcin, osteonectin, osteopontin, proteoglycans, proteolipids, tenascin, and several growth factors. Enamel proteins also have been suggested to be present in cementum but not yet convincingly demonstrated. Two apparently unique cementum molecules, an adhesion molecule (cementum attachment protein) and an insulin-like growth factor have been identified, but further studies are warranted to confirm the existence and function of these molecules. The recent finding that cementoblasts can express the osteoblast-specific membrane protein bone restricted ifitm-like protein (Bril, a member of interferon inducible transmembrane protein family) adds additional support for similarity between these two cells.
Initiation of Cementum Formation
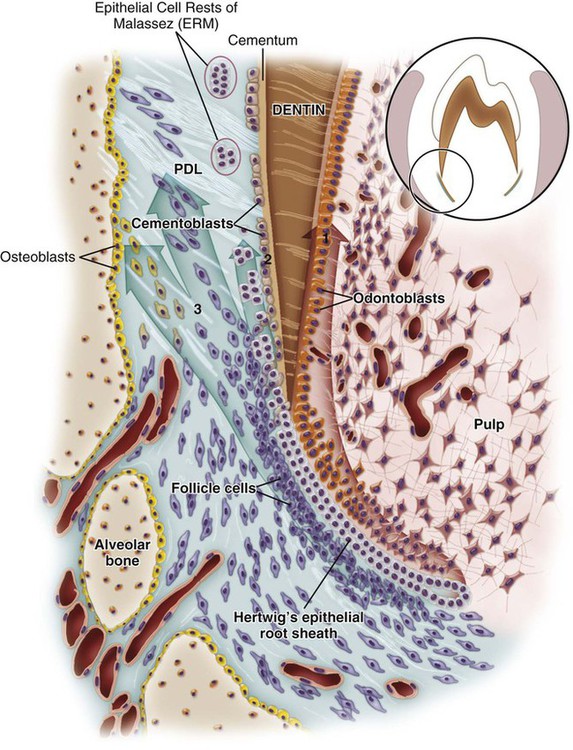
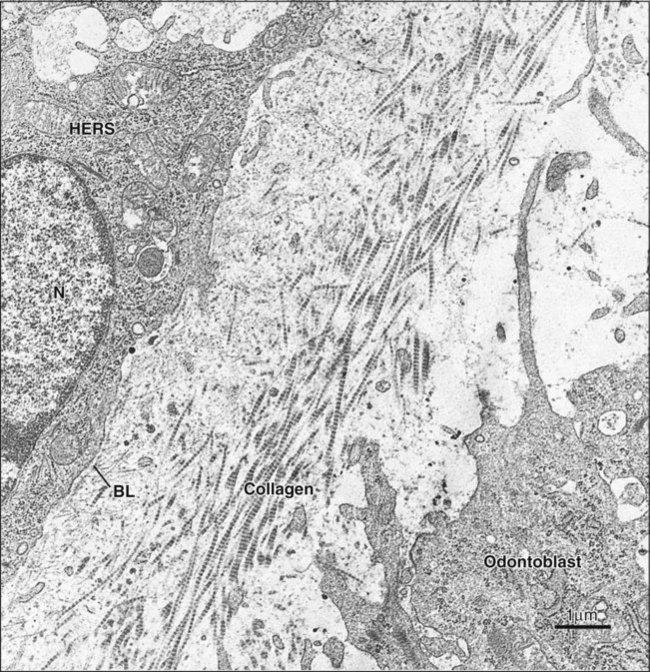
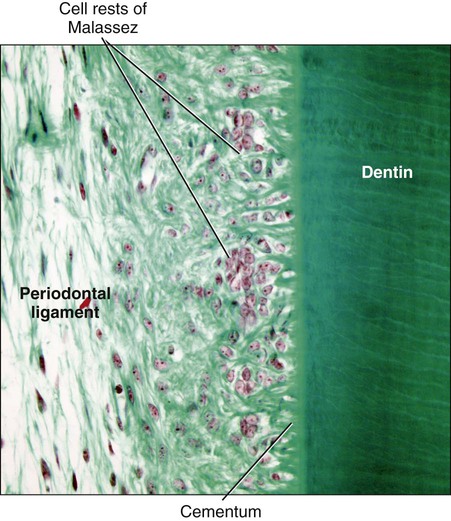
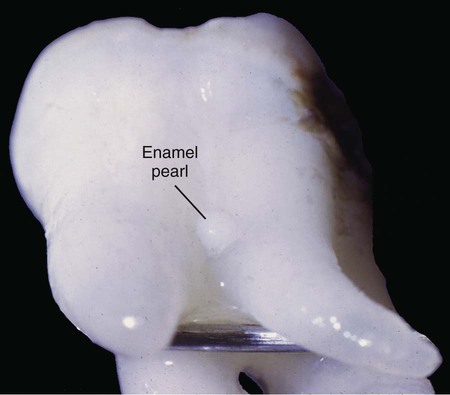
Although cementum formation takes place along the entire root, its initiation is limited to the advancing root edge (Figure 9-3). At this site, Hertwig’s epithelial root sheath (HERS), which derives from the coronoapical extension of the inner and outer enamel epithelium (see Chapter 5), is believed to send an inductive message, possibly by secreting some enamel proteins or other epithelial product, to the facing ectomesenchymal pulp cells. These cells differentiate into odontoblasts and produce a layer of predentin (Figures 9-4 and 9-5). The next series of events results in formation of cementum on the root surface; however, the specific cells and trigger factors responsible for promoting its formation still are unresolved. Current theories include the following: (1) Soon after, HERS becomes interrupted, and ectomesenchymal cells from the inner portion of the dental follicle then can come in contact with the predentin; (2) infiltrating dental follicle cells receive a reciprocal inductive signal from the dentin and/or the surrounding HERS cells and differentiate into cementoblasts; or (3) HERS cells transform into cementoblasts (a process discussed subsequently). During these processes, some cells from the fragmented root sheath form discrete masses surrounded by a basal lamina, known as epithelial cell rests of Malassez, which persist in the mature PDL (Figure 9-6; see also Figure 9-5). Evidence is increasing that these rests are not simply residual cells but instead may participate in maintenance and regeneration of periodontal tissues. If some HERS cells remain attached to the forming root surface, they can produce focal deposits of enamel-like material called enamel pearls (Figure 9-7), most commonly found in the area of furcation of roots.
Origin of Periodontal Cells and Differentiation of Cementoblasts
1. What are the precursors of cementoblasts and PDL fibroblasts?
2. Do cementoblasts express unique genes products, or are they simply positional osteoblasts?
3. Are acellular and cellular cementum phenotypically distinct tissues?
4. What factors promote cementoblast differentiation?
5. What regulates formation of the PDL versus cementogenesis, thus providing a balance between cementum, PDL, and alveolar bone?
Molecular Factors Regulating Cementogenesis
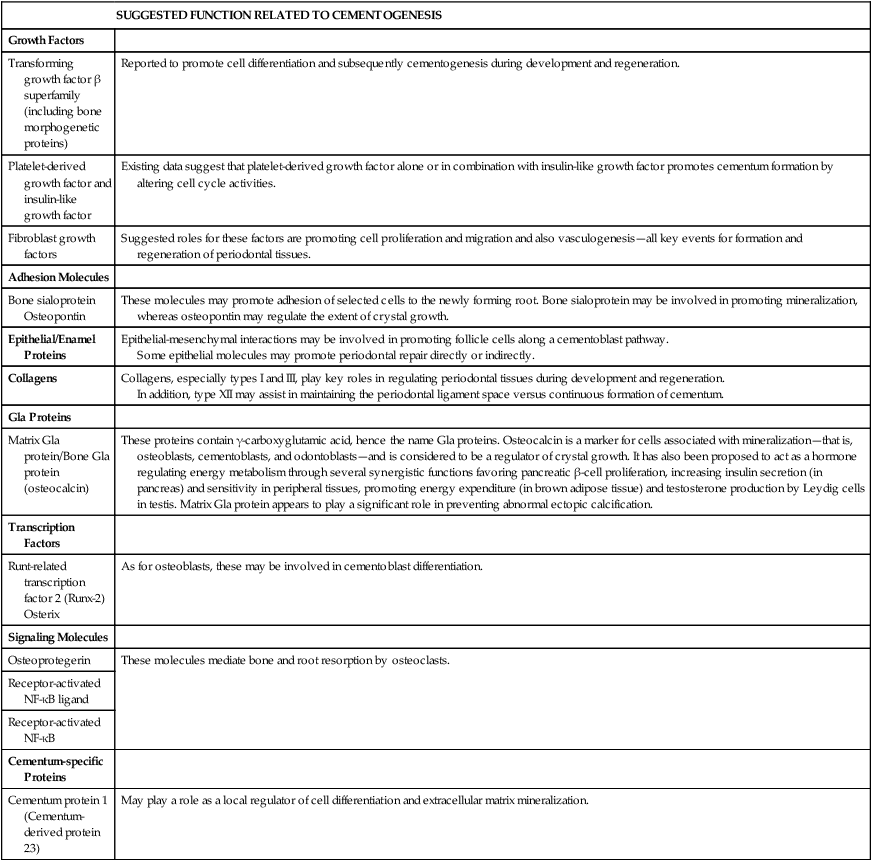
To understand the specific role of phosphate and other molecules, additional studies that focus on defining the cells and factors controlling development, maintenance, and regeneration of periodontal tissues are required. Some of the factors known to be involved in controlling these events are discussed next and are summarized in Table 9-1.
TABLE 9-1
Some Key Molecules in the Periodontium
| SUGGESTED FUNCTION RELATED TO CEMENTOGENESIS | |
| Growth Factors | |
| Transforming growth factor β superfamily (including bone morphogenetic proteins) | Reported to promote cell differentiation and subsequently cementogenesis during development and regeneration. |
| Platelet-derived growth factor and insulin-like growth factor | Existing data suggest that platelet-derived growth factor alone or in combination with insulin-like growth factor promotes cementum formation by altering cell cycle activities. |
| Fibroblast growth factors | Suggested roles for these factors are promoting cell proliferation and migration and also vasculogenesis—all key events for formation and regeneration of periodontal tissues. |
| Adhesion Molecules | |
| Bone sialoprotein Osteopontin |
These molecules may promote adhesion of selected cells to the newly forming root. Bone sialoprotein may be involved in promoting mineralization, whereas osteopontin may regulate the extent of crystal growth. |
| Epithelial/Enamel Proteins | Epithelial-mesenchymal interactions may be involved in promoting follicle cells along a cementoblast pathway. Some epithelial molecules may promote periodontal repair directly or indirectly. |
| Collagens | Collagens, especially types I and III, play key roles in regulating periodontal tissues during development and regeneration. In addition, type XII may assist in maintaining the periodontal ligament space versus continuous formation of cementum. |
| Gla Proteins | |
| Matrix Gla protein/Bone Gla protein (osteocalcin) | These proteins contain γ-carboxyglutamic acid, hence the name Gla proteins. Osteocalcin is a marker for cells associated with mineralization—that is, osteoblasts, cementoblasts, and odontoblasts—and is considered to be a regulator of crystal growth. It has also been proposed to act as a hormone regulating energy metabolism through several synergistic functions favoring pancreatic β-cell proliferation, increasing insulin secretion (in pancreas) and sensitivity in peripheral tissues, promoting energy expenditure (in brown adipose tissue) and testosterone production by Leydig cells in testis. Matrix Gla protein appears to play a significant role in preventing abnormal ectopic calcification. |
| Transcription Factors | |
| Runt-related transcription factor 2 (Runx-2) Osterix |
As for osteoblasts, these may be involved in cementoblast differentiation. |
| Signaling Molecules | |
| Osteoprotegerin | These molecules mediate bone and root resorption by osteoclasts. |
| Receptor-activated NF-κB ligand | |
| Receptor-activated NF-κB | |
| Cementum-specific Proteins | |
| Cementum protein 1 (Cementum-derived protein 23) | May play a role as a local regulator of cell differentiation and extracellular matrix mineralization. |
Epithelial Factors
Epithelial-mesenchymal interactions are required for formation of the tooth crown, and epithelial factors are implicated. The same two populations of cells involved in crown morphogenesis—that is, epithelial and ectomesenchymal cells—also take part in root formation. The possibility that such interactions also are required for development of periodontal tissues and that some of the same signaling molecules are involved is thus a logical assumption. Prospective candidates include enamel proteins, parathyroid hormone-related protein, and basal lamina constituents. In the case of enamel proteins, the debate centers around the fact that enamel proteins have not been detected consistently along forming roots. However, this does not rule out a transient expression at early stages of root formation where they could influence odontoblast and/or cementoblast differentiation. Along this line, an enamel matrix derivative, consisting predominantly of amelogenin molecules, is used clinically to stimulate repair and regeneration, but its mechanism of action remains to be determined (see Chapter 15).
Transcription Factors
As shown in Chapter 6, Runx-2 (runt-related transcription factor 2), also known as Cbfa1 (core binding factor alpha 1), and osterix, downstream from Runx-2, have been identified as master switches for differentiation of osteoblasts. Runx-2 now has been found to be expressed in dental follicle cells, PDL cells, and cementoblasts. Based on similarities between cementoblasts (at least in cellular cementum) and osteoblasts, it is likely that both factors may be involved in cementoblast differentiation. The exact factors triggering expression or activation of these key transcription factors currently are being investigated; BMPs already have been identified as factors promoting expression of Runx-2.
Other Factors
Mineralized tissues such as bone are turning over continually and require a delicate balance between formative and resorptive cells. Two key factors that have emerged as critical to this balance are osteoprotegerin and receptor-activated NF-κB ligand (RANKL). Both are produced by osteoblasts and PDL fibroblasts. As discussed in more detail in Chapter 6, RANKL activates osteoclasts by binding to specific cell surface receptors (RANK), whereas osteoprotegerin acts as a decoy interfering with the binding of RANKL to RANK. Growth factors and cytokines in the local region of the periodontium have been shown to modulate expression of osteoprotegerin and RANKL and thus may be important for controlling osteoclastic-mediated bone and root resorption; thus they may be attractive factors in designing therapeutic agents to regulate the behavior of this cell.
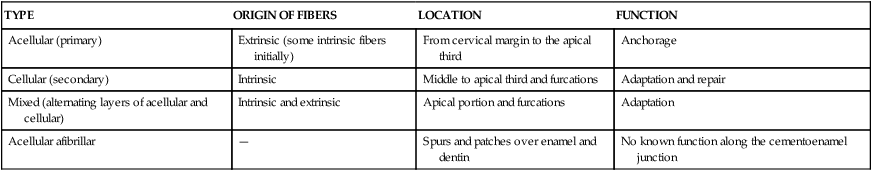
Cementum Varieties
Table 9-2 lists the various types of cementum along with the origin, location, and function of each.
TABLE 9-2
Type, Distribution, and Function of Cementum
| TYPE | ORIGIN OF FIBERS | LOCATION | FUNCTION |
| Acellular (primary) | Extrinsic (some intrinsic fibers initially) | From cervical margin to the apical third | Anchorage |
| Cellular (secondary) | Intrinsic | Middle to apical third and furcations | Adaptation and repair |
| Mixed (alternating layers of acellular and cellular) | Intrinsic and extrinsic | Apical portion and furcations | Adaptation |
| Acellular afibrillar | — | Spurs and patches over enamel and dentin | No known function along the cementoenamel junction |
Acellular Extrinsic Fiber Cementum (Primary Cementum)
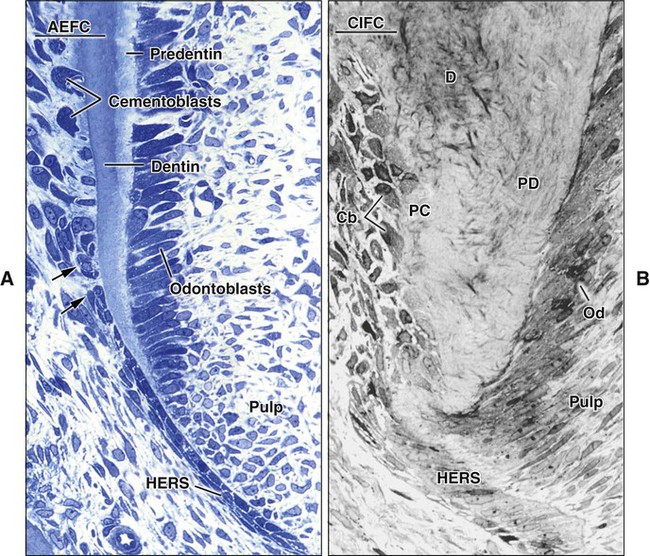
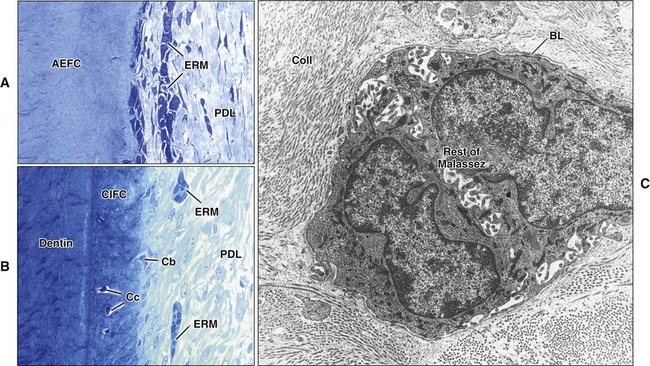
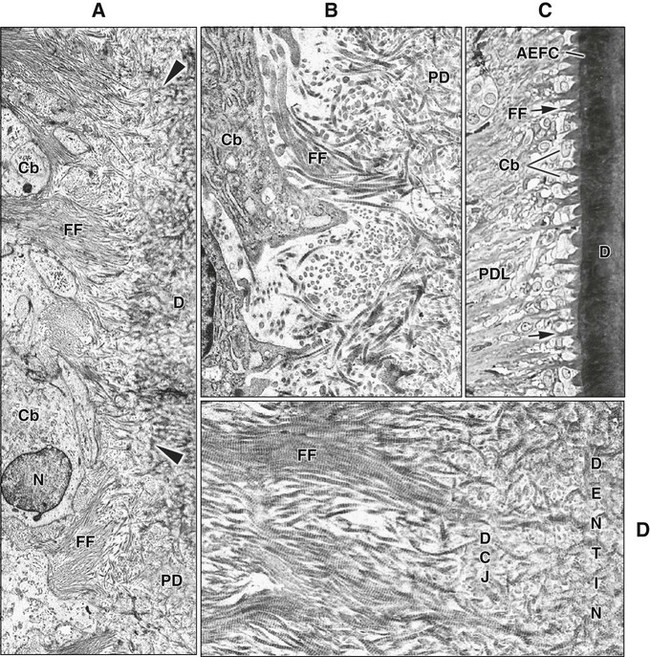
Cementoblasts that produce acellular extrinsic fiber cementum differentiate in proximity to the advancing root edge. During root development in human teeth, the first cementoblasts align along the newly formed but not yet mineralized mantle dentin (predentin) surface following disintegration of HERS (Figure 9-8, A and B). The cementoblasts exhibit fibroblastic characteristics, extend cell processes into the unmineralized dentin, and initially deposit collagen fibrils within it so that the dentin and cementum fibrils intermingle. Mineralization of the mantle dentin starts internally and does not reach the surface until mingling has occurred. Mineralization then spreads across into cementum under the regulatory influence of noncollagenous matrix proteins, thereby establishing the cementodentinal junction. In rodents, initial cementum deposition occurs onto the already mineralized dentin surface, preventing the intermingling of fibers (see Figure 9-3, A).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses