Host Modulation
Host modulation is a relatively new term that has been incorporated into our dental jargon, but it has not been well defined. Host can be defined as “the organism from which a parasite obtains its nourishment,” or in the transplantation of tissue, “the individual who receives the graft.” Modulation is defined as “the alteration of function or status of something in response to a stimulus or an altered chemical or physical environment” (Taber’s Medical Dictionary, 2004). In diseases of the periodontium that are initiated by bacteria, the “host” clearly is the individual who harbors these pathogens; however, it was not clear for many years whether it was possible to modulate the host response to these pathogens and other stimuli leading to the breakdown of the attachment apparatus. Host modulation with chemotherapeutic agents or drugs is the latest adjunctive therapeutic option for the management of periodontal diseases. The concept of host modulation is fairly new to the field of dentistry but is universally understood by most physicians who routinely apply the principles of host modulation to the management of a number of chronic progressive disorders such as arthritis and osteoporosis. The concept of host modulation was first introduced to dentistry by Williams101 and Golub et al35 and then expanded on by many other scholars in the dental profession. In 1990, Williams concluded “there are compelling data from studies in animals and human trials indicating that pharmacologic agents, that modulate the host responses believed to be involved in the pathogenesis of periodontal destruction, may be efficacious in slowing the progression of periodontitis.”101 In 1992, Golub and colleagues discussed “host modulation with tetracyclines and their chemically modified analogues.”35 The future that these authors described has arrived, and to better understand this new era in disease management, we must first look to the pathogenesis of periodontitis.
Many clinicians previously believed that periodontal disease was an inevitable consequence of aging and was uniformly distributed in the population. They thought that disease severity was directly correlated with plaque levels (i.e., the worse the oral hygiene, the worse the periodontal disease) and that disease progression occurred in a continuous, linear manner throughout life. Now, as a result of better epidemiologic data, there has been a paradigm shift in how clinicians and scientists view the prevalence and progression of this common disease. It has been well established that periodontal disease is not a natural consequence of aging and disease severity is not necessarily correlated with plaque levels. Theories about the pathogenesis of periodontitis have evolved from a purely plaque-associated disease to the more recent hypotheses that place considerable emphasis on the host’s response to the bacteria.10 The first Surgeon General’s report on “Oral Health in America,” published in 2000, recognized the importance of dental health in the overall general health and well-being of a patient.93 Recent research findings indicate possible associations between chronic oral infections, such as periodontitis, and systemic disorders, such as diabetes, cardiovascular and lung diseases, stroke, osteoporosis, and rheumatoid arthritis. The Surgeon General’s report assesses these emerging associations and explores factors that may underlie oral-systemic disease connections. Along with these findings and the emergence of the discipline of periodontal medicine, there have been many developments in therapeutic approaches to the management of periodontitis. The development of the chemotherapeutic approach known as “host modulation” required a thorough understanding of the host response and the impact of a variety of risk factors.
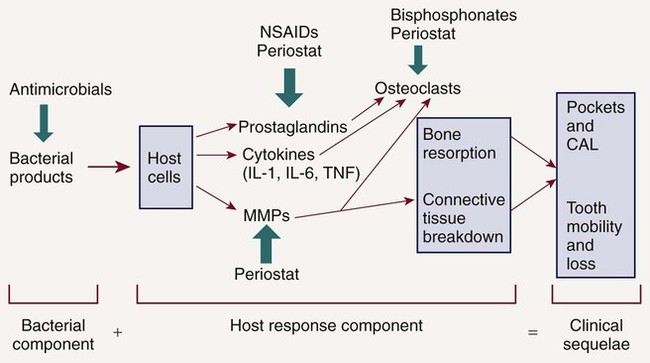
In 1985, research began to focus on bacterial-host interactions.58 It had been recognized that although bacterial pathogens initiate the periodontal inflammation, the host response to these pathogens is equally, if not more, important in mediating connective tissue breakdown, including bone loss. It has become clear that the host-derived enzymes known as the matrix metalloproteinases (MMPs), as well as changes in osteoclast activity driven by cytokines and prostanoids, cause most of the tissue destruction in the periodontium.68 This shift in paradigms, with a focus on the host response, led to the development of host modulatory therapies to improve therapeutic outcomes, slow the progression of disease, and allow for more predictable management of patients with periodontitis. This therapeutic strategy not only may be relevant to individuals at greater risk for periodontal disease but also may be important in the management of those at risk for a number of related systemic conditions. To better understand the host factors that clinicians are attempting to modulate, we need a more detailed assessment of the role of the host response in periodontal pathogenesis (Figure 49-1). After the accumulation of subgingival plaque bacteria, a variety of microbial substances, including chemotactic factors such as lipopolysaccharide (LPS), microbial peptides, and other bacterial antigens, diffuse across the junctional epithelium into the gingival connective tissues. The periodontium is anatomically unique in that the junctional epithelium ends on the tooth surface, which is nonliving tissue; there is no other such discontinuous lining over the entire surface of the body. The dentogingival junction indicates a priori vulnerability to bacterial attack. Epithelial and connective tissue cells are thus stimulated to produce inflammatory mediators that result in an inflammatory response in the tissues. The gingival vasculature dilates (vasodilation) and becomes increasingly permeable to fluid and cells. Fluid accumulates in the tissues, and defense cells migrate from the circulation toward the source of the chemotactic stimulus (bacteria and their products) in the gingival crevice. Neutrophils, or polymorphonuclear leukocytes (PMNs), predominate in the early stages of gingival inflammation to phagocytose and kill plaque bacteria. Bacterial killing by PMNs involves both intracellular mechanisms (after phagocytosis of bacteria within membrane-bound structures inside the cell) and extracellular mechanisms (by release of PMN enzymes and oxygen radicals outside the cell). As bacterial products enter the circulation, committed lymphocytes return to the site of infection, and B lymphocytes are transformed to plasma cells, which produce antibodies against specific bacterial antigens. Antibodies are released in the gingival tissues and, in the presence of complement, facilitate and enhance PMN phagocytosis and bacterial killing.
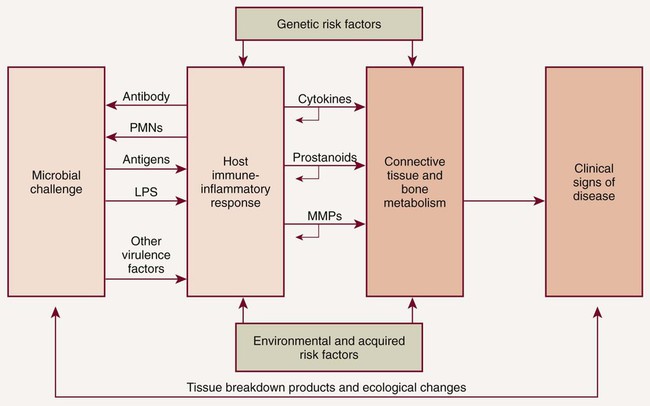
The elevations in the proinflammatory or destructive mediators in response to bacterial challenge are counterbalanced by elevations in antiinflammatory or protective mediators such as the cytokines IL-4 and IL-10, as well as other mediators, such as IL-1ra (receptor antagonist), and tissue inhibitors of metalloproteinases (TIMPs) (Figure 49-2). Under conditions of health, the antiinflammatory or protective mediators serve to control tissue destruction. If there are adequate levels of these antiinflammatory or protective mediators to keep the host response to the bacterial challenge in check, the individual will be disease resistant. If an imbalance occurs, with excessive levels of the proinflammatory or destructive mediators present in the host tissues, tissue destruction will ensue in the susceptible host. Another potential target for host modulation could entail the use of pharmacologic agents, which either mimic or result in elevations of endogenous antiinflammatory or protective mediators.
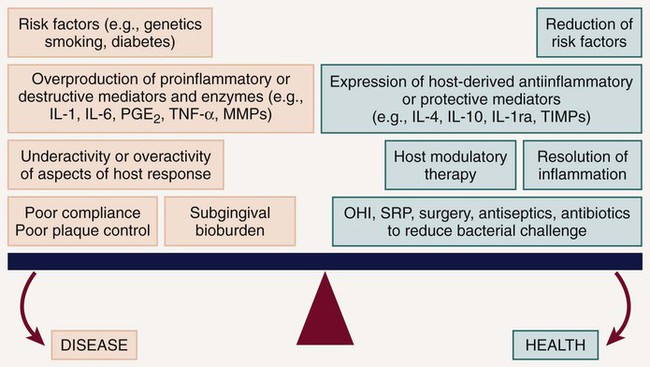
Thus plaque bacteria initiate the disease, and bacterial antigens that cross the junctional epithelium drive the inflammatory process. Therefore bacteria are essential for periodontitis to occur, but they are insufficient by themselves to cause disease. For periodontitis to develop, a susceptible host is also required. The majority of periodontal breakdown (bone loss, attachment loss) is caused by host-derived destructive enzymes (MMPs) and inflammatory mediators (prostaglandins, interleukins) that are released during the cascade of destructive events that occur as part of the inflammatory response71 (see Figure 49-1). Paradoxically, the inflammatory response, which is essentially protective in design, is responsible for much of the breakdown of the soft and hard periodontal tissues. Periodontal disease is characterized by high concentrations of MMPs, cytokines, and prostanoids in the periodontal tissues, whereas periodontal health is characterized by the opposite.70 The purpose of HMT is to restore the balance of proinflammatory or destructive mediators and antiinflammatory or protective mediators to that seen in healthy individuals. Pocket formation occurs as coronal junctional epithelium is broken down and restored at a more apical location. Plaque bacteria then migrate apically along the root surface deeper into the pocket, where the physical conditions favor the proliferation of gram-negative anaerobic species. Bacterial products continue to challenge the host, and the host continues its frustrated response against these bacteria and their products. Inflammation extends further and further apically, more bone is resorbed, and periodontal ligament (PDL) is broken down. The pocket deepens, and the associated attachment and bone loss result in clinical and radiographic signs of periodontitis. Intervention is required to prevent eventual tooth loss and other sequelae of the disease.
The nature of the host response to the presence of plaque is modified by genetic factors (helping to explain why aggressive periodontitis tends to have a familial aggregation) and systemic and environmental factors (e.g., smoking, diabetes, stress). These risk factors may lead to the imbalance between the proinflammatory and antiinflammatory mediators seen in susceptible individuals (see Figure 49-2). Risk factors can affect onset, rate of progression, and severity of periodontal disease, as well as response to therapy24,41,86 (Box 49-1). Risk assessment is extremely important as we now recognize that some of these risk factors can be modified to reduce a patient’s susceptibility to periodontitis. Risk reduction strategies may include smoking cessation, improved control of diabetes, nutritional supplementation, improved oral hygiene, changes in medication, stress management, weight loss, and more frequent dental visits (Box 49-2). The use of chemotherapeutic agents or drugs specifically designed to treat periodontal diseases is emerging to aid as a risk reduction strategy. Intervention in periodontal disease can now include HMT as one of the available adjunctive treatment options. The term adjunctive is meant to imply “in addition to conventional therapies” or “in addition to other established therapies.” For the management of periodontal diseases, conventional approaches were initially mechanical in nature, that is, surgery, as well as scaling and root planing (SRP). Initially, adjunctive therapies were solely antimicrobial such as the use of antiseptics and antibiotics (local and/or systemic). New adjunctive approaches involve modulation of the host response. Researchers are also investigating HMTs, which aim to modify or reduce destructive aspects of the host response so that the immune-inflammatory response to plaque is less damaging to the periodontal tissues. Removal of plaque by SRP targets one aspect of the pathogenic process by reducing the bacterial burden and therefore the antigenic challenge that drives the inflammatory response in the host tissues. However, the bacterial challenge is never completely eliminated after SRP, and recolonization by bacterial species occurs. HMTs offer the potential for downregulating destructive aspects and upregulating protective aspects of the host response so that, in combination with conventional treatments to reduce the bacterial burden, the balance between health (resolution of inflammation and wound healing) and disease progression (continued proinflammatory events) is tipped in the direction of a healing response.
HMT can be used to reduce excessive levels of enzymes, cytokines, and prostanoids and should not reduce levels below constitutive levels. HMTs can also modulate osteoclast and osteoblast function (Figure 49-3) but should not impact normal tissue turnover. HMT is key to addressing many of the risk factors that have adverse effects on the host response, which are either not easily managed (e.g., smoking, diabetes) or cannot be changed (e.g., genetic susceptibility). In addition, host modulatory agents might be used to increase the levels of a person’s own protective or antiinflammatory mediators. Use of systemic HMTs for treatment of a patient’s periodontal condition may also provide benefits for other inflammatory disorders such as arthritis, cardiovascular disease, dermatologic conditions, diabetes, rheumatoid arthritis, and osteoporosis. Also, patients who are currently taking host modulatory agents, such as nonsteroidal antiinflammatory drugs (NSAIDs), bisphosphonates, or tetracyclines, as well as newer agents targeting specific cytokines for the management of medical conditions, may be experiencing periodontal benefits from these systemic medications prescribed for the management of other chronic inflammatory conditions.
Systemically Administered Agents
Nonsteroidal Antiinflammatory Drugs
NSAIDs inhibit the formation of prostaglandins, including PGE2, which is produced by neutrophils, macrophages, fibroblasts, and gingival epithelial cells in response to the presence of LPS, a component of the cell wall of gram-negative bacteria. PGE2 has been extensively studied in periodontal disease because it upregulates bone resorption by osteoclasts.36,47,69 Levels of PGE2 have been shown to be elevated in patients with periodontal disease compared with healthy patients.38,69 PGE2 also inhibits fibroblast function and has inhibitory and modulatory effects on the immune response.39
NSAIDs inhibit prostaglandins and therefore reduce tissue inflammation. They are used to treat pain, acute inflammation, and a variety of chronic inflammatory conditions. NSAIDs include the salicylates (e.g., aspirin), indomethacin, and the propionic acid derivatives (e.g., ibuprofen, flurbiprofen, and naproxen). The ability of NSAIDs to block PGE2 production, thereby reducing inflammation and inhibiting osteoclast activity in the periodontal tissues, has been investigated in patients with periodontitis. Studies have shown that systemic NSAIDs, such as indomethacin,103 flurbiprofen,102 and naproxen46 administered daily for up to 3 years, significantly slowed the rate of alveolar bone loss compared with placebo. However, NSAIDs have some serious disadvantages when considered for use as a HMT for periodontitis. Daily administration for extended periods is necessary for periodontal benefits to become apparent, and NSAIDs are associated with significant side effects, including gastrointestinal problems, hemorrhage (from decreased platelet aggregation), and renal and hepatic impairment. Furthermore, research shows that the periodontal benefits of taking long-term NSAIDs are lost when patients stop taking the drugs, with a return to or even an acceleration of the rate of bone loss seen before NSAID therapy, often referred to as a “rebound effect.”104 For these reasons, the long-term use of NSAIDs as an adjunctive treatment for periodontitis has never really developed beyond research studies.
It was previously anticipated that the selective cyclooxygenase-2 (COX-2) inhibitors may offer promise as adjunctive treatments in the management of periodontitis. The enzyme cyclooxygenase, which converts arachidonic acid to prostaglandins, exists in two functionally distinct isoforms, COX-1 and COX-2. COX-1 is constitutively expressed and has antithrombogenic and cytoprotective functions. Therefore, inhibition of COX-1 by nonselective NSAIDs causes side effects such as gastrointestinal ulceration and impaired hemostasis. COX-2 is induced after stimulation by various cytokines, growth factors, and LPS and results in the production of elevated quantities of prostaglandins. Inhibition of COX-2 by selective COX-2 inhibitors results in reduction of inflammation. Researchers considered that the use of selective COX-2 inhibitors offered the prospect for reducing periodontal inflammation without the side effects typically observed after long-term (nonselective) NSAID therapy, and preliminary studies identified that selective COX-2 inhibitors slowed alveolar bone loss in animal models5,45 and modified prostaglandin production in human periodontal tissues.95 However, the selective COX-2 inhibitors were later identified to be associated with significant and life-threatening adverse effects (i.e., myocardial infarction), resulting in some drugs being withdrawn from the market. In summary, NSAIDs (including the selective COX-2 specific inhibitors) are presently not indicated as adjunctive HMTs in the treatment of periodontal disease.
Bisphosphonates
The bisphosphonates are bone-seeking agents that inhibit bone resorption by disrupting osteoclast activity. Their precise mechanism of action is unclear, but research has shown that bisphosphonates interfere with osteoblast metabolism and secretion of lysosomal enzymes.100 More recent evidence has suggested that bisphosphonates also possess anticollagenase properties.64 The ability of bisphosphonates to modulate osteoclast activity clearly may be useful in the treatment of periodontitis. Research has demonstrated that in naturally occurring periodontitis in beagle dogs, treatment with the bisphosphonate, alendronate, significantly increased bone density compared with placebo.80 In animal models of experimentally induced periodontitis, bisphosphonates reduced alveolar bone resorption.88,100 In human studies, these agents resulted in enhanced alveolar bone status and density.18,82
Some bisphosphonates have the unwanted effects of inhibiting bone calcification and inducing changes in white blood cell counts. Also, there have been recent reports of avascular necrosis of the jaws following bisphosphonate therapy, with the resultant risk of bone necrosis following dental extractions.11 The recent reports of bisphosphonate-related osteonecrosis of the jaw (BRONJ), although primarily associated with intravenous administration of bisphosphonates rather than oral administration, has impeded the development of bisphosphonates as an HMT to manage periodontitis. As with NSAIDs, at present there are no bisphosphonate drugs that are approved and indicated for treatment of periodontal diseases.
Sub-Antimicrobial-Dose Doxycycline
Sub-antimicrobial-dose doxycycline (SDD) is a 20-mg dose of doxycycline (Periostat) that is approved and indicated as an adjunct to SRP in the treatment of chronic periodontitis. It is taken twice daily for 3 months, up to a maximum of 9 months of continuous dosing. The 20-mg dose exerts its therapeutic effect by enzyme, cytokine, and osteoclast inhibition rather than by any antibiotic effect. Research studies have found no detectable antimicrobial effect on the oral flora or the bacterial flora in other regions of the body and have identified clinical benefit when used as an adjunct to SRP. At present, SDD (Periostat) is the only systemically administered HMT specifically indicated for the treatment of chronic periodontitis that is approved by the U.S. Food and Drug Administration (FDA) and accepted by the American Dental Association (ADA). In addition, a modified-release SDD was recently approved by the FDA (Oracea) for the treatment of the common skin disorder rosacea and is routinely prescribed within the dermatology community. Studies conducted by Preshaw et al,79 using this same modified-release SDD versus placebo in 266 subjects with periodontitis as an adjunct to SRP resulted in significantly greater clinical benefits than SRP alone in the treatment of periodontitis. It will be interesting to see what the long-term benefits to oral health will be in rosacea patients being prescribed this new drug. There has also been considerable off-label use of this new modified-release SDD for the treatment of periodontal diseases based on the understanding that once-a-day administration can increase the level of compliance as compared to twice-a-day oral administration.
Locally Administered Agents
Nonsteroidal Antiinflammatory Drugs
Topical NSAIDs have shown benefit in the treatment of periodontitis. One study of 55 patients with chronic periodontitis who received topical ketorolac mouth rinse reported that gingival crevicular fluid (GCF) levels of PGE2 were reduced by approximately half over 6 months and that bone loss was halted.49 In addition, locally administered ketoprofen has been investigated. To date, topically administered NSAIDs have not been approved as local HMTs for the management of periodontitis.
Host Modulation and Comprehensive Periodontal Management
The term periodontal management suggests a much broader concept of periodontal care than the term periodontal treatment. This concept is extremely important considering the chronic nature of the disease. Management includes thorough medical and dental history and examination (clinical charting and radiographs), assessment of risk factors, diagnosis, development of a treatment strategy, initial and definitive treatment planning, review of treatment outcomes and reevaluation, long-term supportive periodontal therapy (maintenance care), and assessment of prognosis. In addition, as new data continue to emerge regarding biochemical assessments of disease activity (measuring levels of proinflammatory mediators, bone and connective tissue breakdown products in the GCF, saliva, and tissues of the oral cavity), new diagnostic and prognostic tests may become part of our established protocols for comprehensive periodontal disease management in the future. However, controlling the bacteria that cause periodontal infections remains a central focus of effective periodontal treatment. Understanding the importance of the host response and the impact of risk factors now allows clinicians to provide complementary treatment strategies simultaneously for their patients (Figure 49-4).
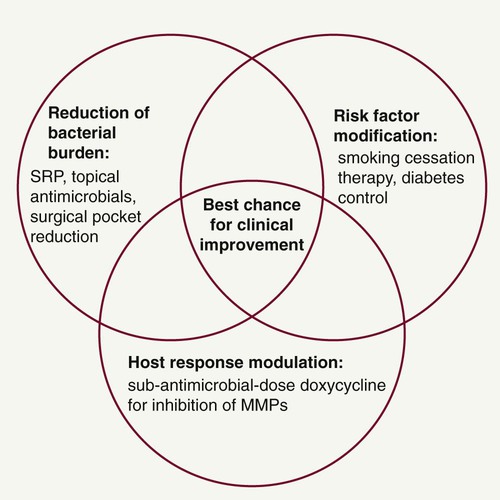
In addition to patient motivation, oral hygiene instruction, and SRP to reduce the bacterial challenge, a key treatment strategy when managing periodontitis patients is risk factor modification. The harmful effects of smoking on the periodontal tissues are well documented,51 and successful smoking cessation therapy will likely be a major benefit to patients with periodontitis. Smoking cessation counseling can be undertaken in the dental office (if staff are appropriately trained) or through collaboration with the patient’s physician or specialized clinics. Given the evidence that smokers have worse periodontal disease than nonsmokers89,105 and that the magnitude and predictability of clinical improvements after treatment are significantly reduced in smokers,1,75 smoking cessation counseling should form a major part of treatment for smokers with periodontitis. Patients with poorly controlled diabetes are also at increased risk for periodontitis,59 and periodontal therapy may have an impact on diabetic control.39 Collaboration with medical colleagues when treating diabetic patients with periodontitis is warranted to ascertain the degree of diabetic control.23 Other possible risks for periodontitis include nonmodifiable factors such as genetics, gender, and race. As the relevance of different risk factors is established through epidemiologic research, clinicians must remain aware of their responsibilities for informing and attempting to change patients’ behaviors in relation to modifiable risks.
• Patient education and motivation, including oral hygiene instruction; use of powered toothbrushes, antiseptics in rinses, toothpastes, and irrigation; and explanation of the rationale for any adjunctive treatments
• Reduction of the bacterial burden by high-quality SRP
• Site-specific antibacterial treatment with local delivery systems or systemic antimicrobial therapy in select cases
• Host response modulation by HMT
It is the responsibility of the dentist to customize the treatment plan for each individual patient by selecting and providing appropriate treatments, following discussion and informed decision making by the patient. Good communication and showing an interest in the patient’s condition are essential to maximize compliance and modify risk factors. The best chance for clinical improvement may come from a combination of targeted treatment approaches for each patient (see Figure 49-4). In fact, Novak et al published very striking improvements in probing depth reductions and clinical attachment level gains in a 6-month, randomized, multicenter, examiner-blinded, placebo-controlled study, which showed that combination therapy of HMT (SDD) plus a locally delivered antimicrobial (doxycycline hyclate gel) and SRP provided optimal improvements in clinical parameters when compared to SRP alone in the treatment of moderate-to-severe periodontitis.66
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses