Anti-infective Therapy
It is well established that the various periodontal diseases are caused by bacterial infection. Bacteria begin reattaching to the crowns of teeth soon after the teeth have been cleaned and begin to form a biofilm. Over time, this supragingival plaque biofilm becomes more complex, which leads to a succession of bacteria that are more pathogenic. Bacteria grow in an apical direction and become subgingival. Eventually, as bone is destroyed, a periodontal pocket is formed. In a periodontal pocket, the bacteria form a highly structured and complex biofilm. As this process continues, the bacterial biofilm extends so far subgingivally that the patient cannot reach it during oral hygiene efforts. In addition, this complex biofilm may now offer some protection from the host’s immunologic mechanisms in the periodontal pocket as well as from antibiotics used for treatment. It has been suggested that an antibiotic strength that is 500 times greater than the usual therapeutic dose may be needed to be effective against bacteria that have become arranged in biofilms.40
It is therefore logical to treat periodontal pockets via the mechanical removal of local factors (including the calculus that harbors bacteria) and also by the disruption of the subgingival plaque biofilm itself. Mechanical removal includes manual instrumentation (e.g., scaling and root planing) and machine-driven instrumentation (e.g., ultrasonic scalers), and these procedures can be considered “anti-infective therapy.” Many chemotherapeutic agents are now available to clinicians who treat periodontal diseases. Systemic anti-infective therapy (oral antibiotics) and local anti-infective therapy (placing anti-infective agents directly into the periodontal pocket) can reduce the bacterial challenge to the periodontium. It is also possible that systemically administered nonsteroidal anti-inflammatory agents may play a role in future adjunctive therapy.68,93
Bacteria and their toxic products cause a loss of attachment and a loss of bone. Ultimately, however, the host’s own immunologic response to this bacterial infection can cause even more bone destruction (i.e., indirect bone loss) than that caused by pathogenic bacteria and their by-products. This immunologic response can be influenced by environmental (e.g., tobacco use), acquired (e.g., systemic disease), and genetic risk factors.77 Chemotherapeutic agents can modulate the host’s immune response to bacteria and reduce the host’s self-destructive immunologic response to bacterial pathogens, thereby reducing bone loss.71,72,75 It is also incumbent on health care providers to counsel patients about the detrimental effects of systemic factors, including medications, stress, and tobacco use.40
It is important to note that significant work has been performed with the use of a systematic evidence-based approach to evaluate the various anti-infective and host modulation therapies99 (see Chapters 78 and 79). The meta-analysis of similar research studies has given power to statistical analysis to evaluate anti-infective chemotherapeutic agents for the treatment of periodontal diseases. Unfortunately, a standardized research protocol has not yet been implemented. As a result, some studies—although relevant—have not been used in the evidence-based approach because of their study design. Further evidence-based and similar research is needed to define protocols more precisely for the use of anti-infective agents to treat periodontal diseases.
Definitions
An anti-infective agent is a chemotherapeutic agent that acts by reducing the number of bacteria present. An antibiotic is a naturally occurring, semisynthetic, or synthetic type of anti-infective agent that destroys or inhibits the growth of selective microorganisms, generally at low concentrations. An antiseptic is a chemical antimicrobial agent that can be applied topically or subgingivally to mucous membranes, wounds, or intact dermal surfaces to destroy microorganisms and to inhibit their reproduction or metabolism. In dentistry, antiseptics are widely used as the active ingredient in anti-plaque and anti-gingivitis oral rinses and dentifrices. Disinfectants (a subcategory of antiseptics) are antimicrobial agents that are generally applied to inanimate surfaces to destroy microorganisms.21
Anti-infective agents can be administered locally or orally. When administered orally, many of these agents can be found in gingival crevicular fluid (GCF). With either approach, their purpose is to reduce the number of bacteria present in the diseased periodontal pocket. The systemic administration of antibiotics may be a necessary adjunct for the controlling of bacterial infection, because bacteria can invade periodontal tissues, thereby making mechanical therapy alone sometimes ineffective.4,18,20,31,76 The local administration of anti-infective agents, generally directly to the pocket, has the potential to provide greater concentrations directly to the infected area and thus reduce possible systemic side effects.
A single chemotherapeutic agent can also have a dual mechanism of action. For example, tetracyclines (especially doxycycline) are chemotherapeutic agents that can reduce collagen and bone destruction via their ability to inhibit the enzyme collagenase. As antibiotic agents, they can also reduce periodontal pathogens in periodontal tissues.20
Systemic Administration of Antibiotics
Background and Rationale
The treatment of periodontal diseases is based on the infectious nature of these diseases (Table 48-1). Ideally, the causative microorganisms should be identified, and the most effective agent should be selected with the use of antibiotic-sensitivity testing. Although this appears simple, the difficulty lies primarily in identifying the specific etiologic microorganisms rather than the microorganisms that are simply associated with various periodontal disorders.19,20
TABLE 48-1
Antibiotics Used to Treat Periodontal Diseases
| Category | Agent | Major Features |
| Penicillin* | Amoxicillin | Extended spectrum of antimicrobial activity; excellent oral absorption; used systemically |
| Augmentin† | Effective against penicillinase-producing microorganisms; used systemically | |
| Tetracyclines | Minocycline | Effective against a broad spectrum of microorganisms; used systemically and applied locally (subgingivally) |
| Doxycycline | ||
| Tetracycline | Effective against a broad spectrum of microorganisms; used systemically and applied locally (subgingivally) | |
| Chemotherapeutically used in subantimicrobial doses for host modulation (Periostat) | ||
| Effective against a broad spectrum of microorganisms | ||
| Quinolone | Ciprofloxacin | Effective against gram-negative rods; promotes health-associated microflora |
| Macrolide | Azithromycin | Concentrates at sites of inflammation; used systemically |
| Lincomycin derivative | Clindamycin | Used in penicillin-allergic patients; effective against anaerobic bacteria; used systemically |
| Nitroimidazole‡ | Metronidazole | Effective against anaerobic bacteria; used systemically and applied locally (subgingivally) as gel |
*Indications: localized aggressive periodontitis, generalized aggressive periodontitis, medically related periodontitis, and refractory periodontitis.
†Amoxicillin and clavulanate potassium.
‡Indications: localized aggressive periodontitis, generalized aggressive periodontitis, medically related periodontitis, refractory periodontitis, and necrotizing ulcerative periodontitis.
An ideal antibiotic for use in the prevention and treatment of periodontal diseases should be specific for periodontal pathogens, allogenic, nontoxic, substantive, not in general use for the treatment of other diseases, and inexpensive.32 Currently, however, an ideal antibiotic for the treatment of periodontal diseases does not exist.49 Although oral bacteria are susceptible to many antibiotics, no single antibiotic at the concentrations achieved in body fluids inhibits all putative periodontal pathogens.97 Indeed, a combination of antibiotics may be necessary to eliminate all putative pathogens from some periodontal pockets70 (Table 48-2).
TABLE 48-2
Common Antibiotic Regimens Used to Treat Periodontal Diseases*
| Regimen | Dosage/Duration | |
| Single Agent | ||
| Amoxicillin | 500 mg | Three times daily for 8 days |
| Azithromycin | 500 mg | Once daily for 4 to 7 days |
| Ciprofloxacin | 500 mg | Twice daily for 8 days |
| Clindamycin | 300 mg | Three times daily 10 days |
| Doxycycline or minocycline | 100 mg to 200 mg | Once daily for 21 days |
| Metronidazole | 500 mg | Three times daily for 8 days |
| Combination Therapy | ||
| Metronidazole + amoxicillin | 250 mg of each | Three times daily for 8 days |
| Metronidazole + ciprofloxacin | 500 mg of each | Twice daily for 8 days |
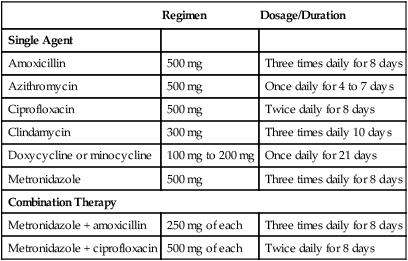
*These regimens are prescribed after a review of the patient’s medical history, periodontal diagnosis, and antimicrobial testing. Clinicians must consult pharmacology references such as Mosby’s GenRx67 or the manufacturer’s guidelines for warnings, contraindications, and precautions.
Data from Jorgensen MG, Slots J: Compend Contin Educ Dent 21:111, 2000.
As always, the clinician—in concert with the patient—must make the final decision regarding any treatment. Thus, the treatment of the individual patient must be based on the patient’s clinical status, the nature of the colonizing bacteria, the ability of the agent to reach the site of infection, and the risks and benefits associated with the proposed treatment plan. The clinician is responsible for choosing the correct antimicrobial agent. Some adverse reactions include allergic or anaphylactic reactions, superinfections of opportunistic bacteria, the development of resistant bacteria, interactions with other medications, upset stomach, nausea, and vomiting.6 Most adverse reactions take the form of gastrointestinal upset.49 Other concerns include the cost of the medication and the patient’s willingness and ability to comply with the proposed therapy.
No consensus exists regarding the magnitude of the risk for the development of bacterial resistance. The common and indiscriminate use of antibiotics worldwide has contributed to increasing numbers of resistant bacterial strains over the last 15 to 20 years, and this trend is likely to continue given the widespread use of antibiotics.96 The overuse, misuse, and widespread prophylactic application of anti-infective drugs are some of the factors that have led to the emergence of resistant microorganisms. Increasing levels of resistance of subgingival microflora to antibiotics has been correlated with the increased use of antibiotics in individual countries.91 However, researchers have noted that the subgingival microflora tends to revert to similar proportions of antibiotic-resistant isolates 3 months after therapy.29,43
Tetracyclines
Tetracyclines have been widely used for the treatment of periodontal diseases. They have been frequently used to treat refractory periodontitis, including localized aggressive periodontitis (LAP)100 (see Table 48-1). Tetracyclines have the ability to concentrate in the periodontal tissues and to inhibit the growth of Aggregatibacter actinomycetemcomitans. In addition, tetracyclines exert an anti-collagenase effect that can inhibit tissue destruction and that may help with bone regeneration.17,59,95
Pharmacology.
The tetracyclines are a group of antibiotics that are produced naturally from certain species of Streptomyces or that are derived semi-synthetically. These antibiotics are bacteriostatic, and they are effective against rapidly multiplying bacteria. They generally are more effective against gram-positive bacteria than gram-negative bacteria. Tetracyclines are effective for the treatment of periodontal diseases in part because their concentration in the gingival crevice is 2 to 10 times that found in serum.2,7,37 This allows a high drug concentration to be delivered into the periodontal pockets. In addition, several studies have demonstrated that tetracyclines at a low GCF concentration (i.e., 2 µg/ml to 4 µg/ml) are very effective against many periodontal pathogens.8,9
Clinical Use.
Tetracyclines have been investigated as adjuncts for the treatment of LAP.82 A. actinomycetemcomitans is a frequent microorganism that is associated with LAP, and it invades tissue. Therefore, the mechanical removal of calculus and plaque from root surfaces may not eliminate this bacterium from the periodontal tissues. Systemic tetracycline can eliminate tissue bacteria, and it has been shown to arrest bone loss and to suppress A. actinomycetemcomitans levels in conjunction with scaling and root planing.81 This combination therapy allows for the mechanical removal of root surface deposits and for the elimination of pathogenic bacteria from within the tissues. Increased posttreatment bone levels have been noted with the use of this method.85 As a result of increased resistance to tetracyclines, metronidazole or amoxicillin with metronidazole has been found to be more effective for the treatment of aggressive periodontitis in children. Some investigators think that metronidazole in combination with amoxicillin–clavulanic acid is the preferable antibiotic.94
The long-term use of low antibacterial doses of tetracyclines has been advocated in the past. One long-term study of patients taking low doses of tetracycline (i.e., 250 mg/day for 2 to 7 years) demonstrated the persistence of deep pockets that did not bleed after probing. These sites contained high proportions of tetracycline-resistant, gram-negative rods (Fusobacterium nucleatum). After the antibiotic was discontinued, the flora was characteristic of sites with disease.49 Therefore, it is not advisable to prescribe long-term regimens of tetracyclines because of the possible development of resistant bacterial strains.55 Although tetracyclines were often used in the past as anti-infective agents, especially for LAP and other types of aggressive periodontitis, they are now frequently replaced by more effective combination antibiotics.49
Specific Agents.
Minocycline.
Minocycline is effective against a broad spectrum of microorganisms. In patients with adult periodontitis, it suppresses spirochetes and motile rods as effectively as scaling and root planing, with suppression evident up to 3 months after therapy. Minocycline can be given twice daily, thereby facilitating compliance as compared with tetracycline. Although it is associated with less phototoxicity and renal toxicity than tetracycline, minocycline may cause reversible vertigo. Minocycline administered at a dose of 200 mg/day for 1 week results in a reduction in total bacterial counts, the complete elimination of spirochetes for up to 2 months, and the improvement of all clinical parameters.21,24
Doxycycline.
Doxycycline has the same spectrum of activity as minocycline, and it may be equally as effective.20 Because doxycycline can be given only once daily, patients may be more compliant. Compliance is also favored because its absorption from the gastrointestinal tract is only slightly altered by calcium, metal ions, or antacids, as is absorption of other tetracyclines. Side effects are similar to those of tetracycline hydrochloride; however, it is the most photosensitizing agent in the tetracycline category.
The recommended dosage when doxycycline is used as an anti-infective agent is 100 mg twice daily the first day, which is then reduced to 100 mg daily. To reduce gastrointestinal upset, 50 mg can be taken twice daily after the initial dose. When given as a sub-antimicrobial dose (to inhibit collagenase), 20 mg of doxycycline twice daily is recommended.17,26 Periostat (CollaGenex Pharmaceuticals Inc, Newtown, PA) and generic forms are currently available for a dose of 20 mg of doxycycline.
Metronidazole
Pharmacology.
Metronidazole is a nitroimidazole compound that was developed in France to treat protozoal infections. It is bactericidal to anaerobic organisms, and it is thought to disrupt bacterial DNA synthesis in conditions with a low reduction potential. Metronidazole is not the drug of choice for treating A. actinomycetemcomitans infections. However, metronidazole is effective against A. actinomycetemcomitans when it is used in combination with other antibiotics.69,70 Metronidazole is also effective against anaerobes such as Porphyromonas gingivalis and Prevotella intermedia.39
Clinical Use.
Metronidazole has been used clinically to treat gingivitis, acute necrotizing ulcerative gingivitis, chronic periodontitis, and aggressive periodontitis. It has been used as monotherapy and also in combination with both root planing and surgery or with other antibiotics. Metronidazole has been used successfully to treat necrotizing ulcerative gingivitis.61
Studies in humans have demonstrated the efficacy of metronidazole for the treatment of gingivitis and periodontitis.60 A single dose of metronidazole (250 mg orally) appears in both serum and GCF in sufficient quantities to inhibit a wide range of suspected periodontal pathogens. When it is administered systemically (i.e., 750 mg/day to 1000 mg/day for 2 weeks), metronidazole reduces the growth of anaerobic flora, including spirochetes, and it decreases the clinical and histopathologic signs of periodontitis.60 The most common regimen is 250 mg three times daily for 7 days.61 Currently, the critical level of spirochetes that is needed to diagnose an anaerobic infection, the appropriate time to give metronidazole, and the ideal dosage or duration of therapy are unknown.39 As monotherapy (i.e., with no concurrent root planing), metronidazole is inferior and at best only equivalent to root planing. Therefore, if it is used, metronidazole should not be administered as monotherapy.
Soder and colleagues83 demonstrated that metronidazole was more effective than placebo for the management of sites that were unresponsive to root planing. Nevertheless, many patients still had sites that bled with probing, despite metronidazole therapy. The existence of refractory periodontitis as a diagnostic consideration indicates that some patients do not respond to conventional therapy, which may include root planing, surgery, or both.
Side Effects.
Metronidazole has an Antabuse effect when alcohol is ingested. The response is generally proportional to the amount ingested, and it can result in severe cramps, nausea, and vomiting. Products that contain alcohol should be avoided during therapy and for at least 1 day after therapy is discontinued. Metronidazole also inhibits warfarin metabolism. Patients who are undergoing anticoagulant therapy should avoid metronidazole, because it prolongs prothrombin time.61 It also should be avoided in patients who are taking lithium. This drug produces a metallic taste in the mouth, which may affect compliance.
Penicillins
Pharmacology.
Amoxicillin–Clavulanate Potassium.
The combination of amoxicillin with clavulanate potassium makes this anti-infective agent resistant to penicillinase enzymes produced by some bacteria. Amoxicillin with clavulanate (Augmentin) may be useful for the management of patients with LAP or refractory periodontitis.68 Bueno and colleagues13 reported that Augmentin arrested alveolar bone loss in patients with periodontal disease that was refractory to treatment with other antibiotics, including tetracycline, metronidazole, and clindamycin.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


