Cytoskeleton, Cell Junctions, Fibroblasts, and Extracellular Matrix
Cytoskeleton
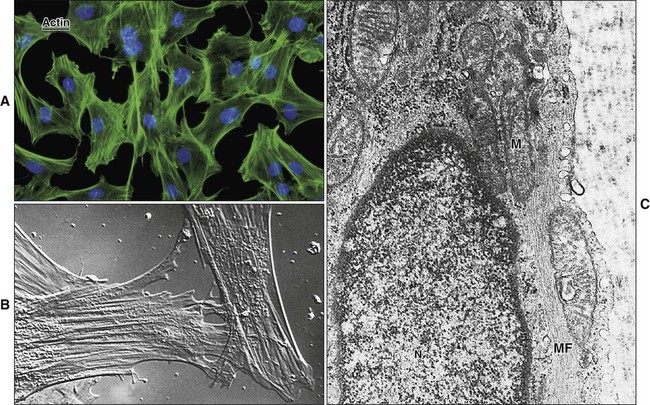
Microfilaments are 6 to 8 nm in diameter and consist of globular actin molecules polymerized into long filaments (Figure 4-1). Microfilaments form tracks for the movement of myosin and serve as intracellular “muscles” for maintenance of cell shape, movement, and contractility. Microfilament networks, along with actin-binding and actin-bundling proteins, are found in association with adhesive cell junctions, as a “web” beneath cell membranes, especially the apical membrane, and as the structural “core” of microvilli, filopodia, and lamelipodia. Actin interacts with the other two components of the cytoskeleton.
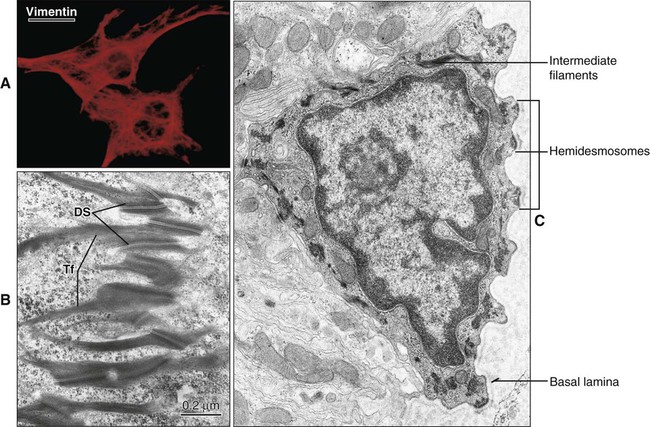
Intermediate filaments are approximately 10 nm in diameter and have a diverse protein composition. They are not contractile but are important in the maintenance of cell shape and contact between adjacent cells and the extracellular matrix. In cells of mesenchymal origin, such as fibroblasts and osteoblasts, intermediate filaments are polymers of the protein vimentin (Figure 4-2). In epithelial cells, intermediate filaments consist of cytokeratins. The filaments form bundles, called tonofilaments, which anchor onto desmosomes (Figure 4-2, B and C). Cytokeratins are a multigene family of proteins made up of basic and more acidic proteins. Cytokeratins occur as linked acidic and basic pairs with differing combinations in different types of epithelia. Their expression patterns have been used to determine the relationship between cell types and as an indication of the origin of various tumors.
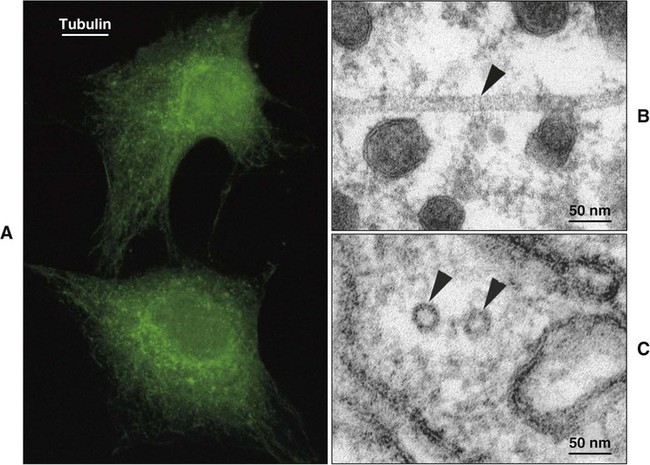
Microtubules are tubular or cylindrical structures with an average diameter of 25 nm (Figure 4-3). Microtubules are composed of the protein tubulin arranged in rings stacked end to end, making up the tubules. Microtubules provide internal support for the cell; are the basis of motility for certain organelles, such as cilia; act as guide paths and part of the motor mechanism for the movement of secretory vesicles and other organelles; and serve to position certain organelles within the cell.
Intercellular Junctions
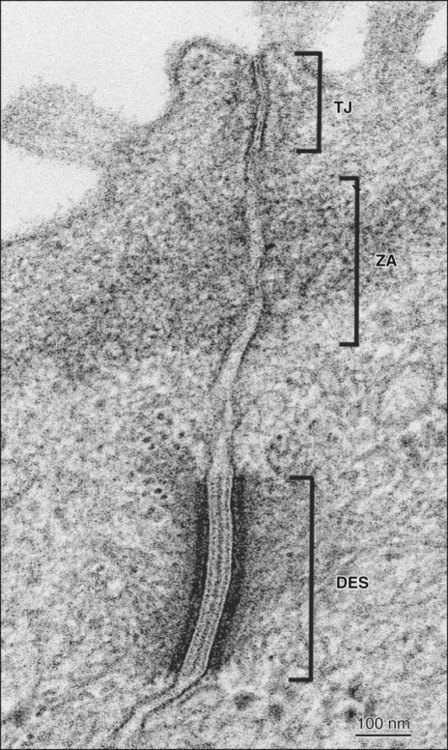
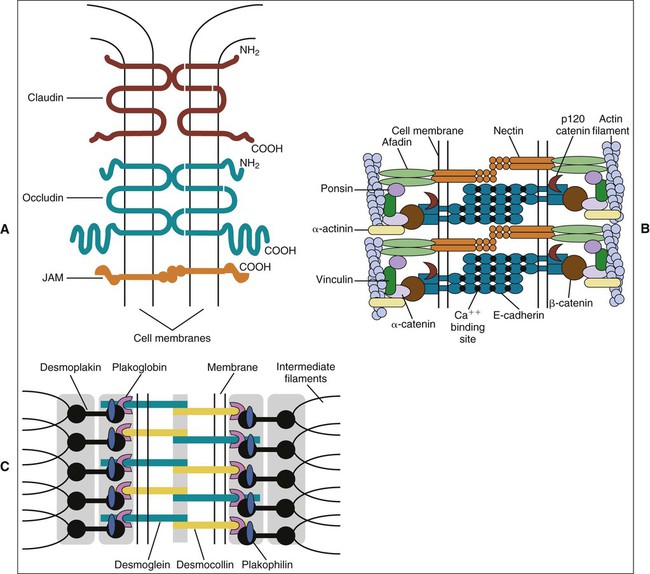
The term zonula describes a junction that completely encircles the cell; macula indicates a junction that is more circumscribed in extent (e.g., patchlike). Junctions may occur in certain combinations. A junctional complex, present between cells of a simple or pseudostratified epithelium, usually consists of a tight junction, a zonula adherens, and desmosomes (Figure 4-4). On the molecular level, intercellular junctions typically consist of three components: a transmembrane adhesive protein, a cytoplasmic adapter protein, and a cytoskeletal filament. These three components differ depending on the type of junction.
In occluding, or tight junctions (Figure 4-5, A; see also Figure 4-4), the opposing cell membranes are held in close contact by the presence of transmembrane adhesive proteins arranged in anastomosing strands that encircle the cell. The intercellular space essentially is obliterated at the tight junction. The transmembrane adhesive proteins—which include occludin, members of the claudin family, and in some tissues, junctional adhesion molecule—interact homotypically with the same proteins on the adjacent cell. Several cytoplasmic proteins associate with the intracellular portions of the transmembrane proteins; these include cell polarity–related proteins, vesicular transport–related proteins, kinases, transcription factors, and a tumor suppressor protein. In addition, some of the cytoplasmic proteins of the tight junctions bind to actin filaments. Tight junctions control the passage of material through the intercellular spaces (e.g., from the interstitium to the lumen of a gland). They also have an important role as a “fence” to define and maintain the two major domains of the cell membrane, the apical and basolateral surfaces. The “tightness” of the junction to water and ions (especially cations) is related to the specific claudin(s) present and is correlated with the number of strands of transmembrane proteins. For example, tight junctions joining salivary gland secretory cells have only two or three junctional strands and are relatively permeable to water, whereas those joining salivary gland striated duct cells may have six to nine strands and are relatively impermeable to water. The permeability of tight junctions in some tissues may be regulated by certain neurotransmitters and hormones.
Adhesive junctions hold cells together or anchor cells to the extracellular matrix. In contrast to tight junctions, the intercellular space in cell-cell adhesive junctions is maintained at approximately 20 nm. Adhesive junctions also are important in cellular signaling. Their cytoplasmic components may interact with the cytoskeleton, triggering changes in cell shape or motility, or with certain tumor suppressor molecules, or they may act as nuclear transcription factors or coactivators. In some instances, the loss of cell-cell or cell-matrix contact may lead to apoptosis (programmed cell death), whereas in others, loss of contact may lead to loss of cell polarity and differentiation or unregulated cell proliferation. In cell-cell adhesive junctions the principal transmembrane proteins are members of the cadherin family. Cadherins are calcium ion–dependent proteins that interact homotypically with cadherins on the adjacent cell. The cytoplasmic adapter proteins are members of the catenin family. Catenins interact with the cytoplasmic domain of the transmembrane cadherin molecule, with the cytoskeleton, and with a number of other proteins, including kinases, and tumor suppressor molecules that are associated with adhesive junctions. In the zonula adherens (see Figures 4-4 and 4-5, B), the cadherin family member is E-cadherin, α- and β-catenin are the cytoplasmic adapters, and actin filaments are the cytoskeletal component. The catenins and actin filaments are concentrated on the cytoplasmic side of the cell membrane at the zonula adherens to form a dense web that is continuous with the terminal web of actin at the apical (and sometimes the basal) end of the cells. Another transmembrane adhesive protein present in the adherens junction is nectin, a member of the immunoglobulin superfamily. Nectin has an important role during junction formation, establishing the initial adhesion site and recruiting E-cadherin and other proteins to the junction. Other cytoplasmic proteins associated with the zonula adherens include p120 catenin, a signaling molecule associated with E-cadherin that is important in stabilizing the junction; afadin, which links nectin to the actin cytoskeleton; vinculin and α-actinin, which are actin-binding proteins; and ponsin, which links afadin and vinculin (see Figure 4-5, B).
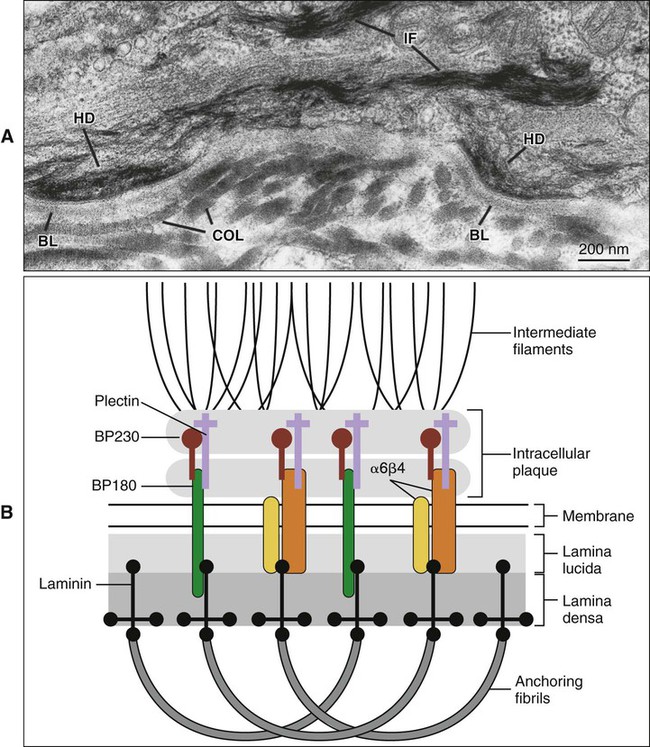
Hemidesmosomes link the cell to the basal lamina and, through additional extracellular molecules, to the rest of the extracellular matrix. The transmembrane adhesive molecules present in hemidesmosomes (Figure 4-6) are the integrin α6β4, which binds specifically to the basal lamina glycoprotein laminin, and collagen XVII (also identified as BP180). Like in the desmosome, the cytoplasmic adapter proteins, bullous pemphigoid antigen 230 (BP230) and plectin, form a dense plaque on the cytoplasmic surface of the hemidesmosome, which functions as an attachment site for intermediate filaments.
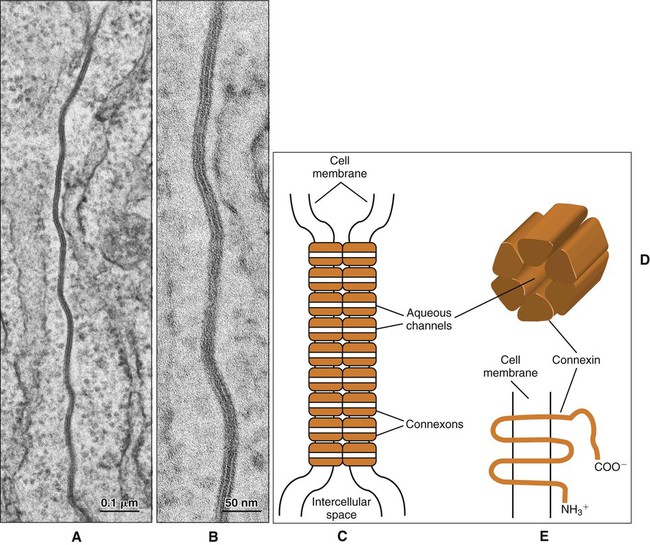
Gap junctions are plaque-like regions of the cell membrane where the intercellular space narrows to 2 to 3 nm and transmembrane proteins of the connexin family form aqueous channels between the cytoplasm of adjacent cells (Figure 4-7). These proteins have specific tissue and cellular distributions and confer differing permeability properties to the gap junctions. Six connexin molecules form a connexon, which has a central channel approximately 2 nm in diameter (Figure 4-7, D). The connexons in one cell pair with connexons in the adjacent cell to create a patent channel. Small molecules, such as ions and signaling molecules, can move readily from one cell to another. Gap junctions electrically couple cells and allow for a coordinated response to a stimulus by the cells that are interconnected.
Epithelium–Connective Tissue Interface
The basal lamina has an overall thickness of 50 to 100 nm and consists of two structural components, the lamina lucida, adjacent to the basal cell membrane, and the lamina densa, between the lamina lucida and the connective tissue (Figure 4-8). In epithelia, there is a third layer, the lamina fibroreticularis, closely associated with the lamina densa. The main constituents of the basal lamina are type IV collagen, which forms a “chicken-wire” network; the adhesive glycoprotein laminin; and a heparan sulfate proteoglycan. Fibronectin, an adhesive glycoprotein, type III collagen (reticular fibers), type VII collagen (anchoring fibrils), and other types of collagen all made by fibroblasts are present in the lamina fibroreticularis and help maintain the attachment of the basal lamina to the underlying connective tissue. There exists also a special, atypical basal lamina between the ameloblasts and maturing enamel (see Chapter 7), and between the gingival and the tooth surface (see Chapter 12).
Fibroblasts
Cellular Organization
Fibroblasts usually are recognized by their association with collagen fiber bundles (Figures 4-9 and 4-10). The resting fibroblast is an elongated cell with little cytoplasm and a dark-staining, flattened nucleus containing condensed chromatin (see Figure 4-9). Active fibroblasts have an oval-shaped, pale-staining nucleus and a greater amount of cytoplasm (see Figure 4-9). The degree of synthetic and secretory capacity of fibroblasts is evidenced by the amount of rough endoplasmic reticulum, secretory granules, and mitochondria, and the extent of the Golgi complex in their cytoplasm (see Figure 4-10)/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses