Periodontal Therapy in the Female Patient
Puberty
Puberty occurs between the average ages of 11 to 14 in most women. The production of sex hormones (estrogen and progesterone) increases, then remains relatively constant during the remainder of the reproductive phase. Also, the prevalence of gingivitis increases, without an increase in the amount of plaque. Gram-negative anaerobes, especially Prevotella intermedia, have been implicated in association with puberty gingivitis. Kornman and Loesche57 postulated that this anaerobic organism may use ovarian hormone as a substitute for vitamin K growth factor. Levels of black-pigmented Bacteroides, especially P. intermedia (formerly known as Bacteroides intermedius), are thought to increase with increased levels of gonadotropic hormones in puberty. Capnocytophaga species also increase in incidence, as well as in proportion. These organisms have been implicated in the increased bleeding tendency observed during puberty.
Recent studies associated with pubertal gingivitis indicate proportionately elevated motile rods, spirochetes, and P. intermedia.81 Statistically significant increases in gingival inflammation and in the proportions of P. intermedia and Prevotella nigrescens have been seen in pubertal gingivitis.85 A recent study of 11- to 17-year-old adolescents found higher levels of Actinobacillus actinomycetemcomitans and Fusobacterium nucleatum, which were associated with bleeding indices, probing depth, and attachment loss.70
During the reproductive years, women tend to have a more vigorous immune response, including higher immunoglobulin concentrations, stronger primary and secondary responses, increased resistance to the induction of immunologic tolerance, and a greater ability to reject tumors and homografts.116 Allergy, sensitivity, and asthma occur more often in young men, but after puberty, women become more susceptible than their male counterparts.
Management
During puberty, education of the parent or caregiver is part of successful periodontal therapy. Preventive care, including a vigorous program of oral hygiene, is also vital.5 Milder gingivitis cases respond well to scaling and root planing, with frequent oral hygiene reinforcement. Severe cases of gingivitis may require microbial culturing, antimicrobial mouthwashes and local site delivery, or antibiotic therapy. Periodontal maintenance appointments may need to be more frequent when periodontal instability is noted.
The clinician should recognize the periodontal manifestations and/or intraoral lesions reflected by systemic diseases (i.e., diabetes).24,92 Thorough review of the patient’s medical history and medical referral should occur when deemed necessary. The clinician should be aware of the effects of chronic regurgitation of gastric contents on intraoral tissues; this age group also is susceptible to eating disorders, namely, bulimia and anorexia nervosa. Perimolysis (smooth erosion of enamel and dentin), typically on the lingual surfaces of maxillary anterior teeth, varies with the duration and frequency of the behavior.16 Also, enlargement of the parotid glands (occasionally sublingual glands) has been estimated to occur in 10% to 50% of patients who “binge and purge.”75 Therefore a diminished salivary flow rate may also be present, which will increase oral mucous membrane sensitivity, gingival erythema, and caries susceptibility.
Menses
Periodontal Manifestations
During the reproductive years, the ovarian cycle is controlled by the anterior pituitary gland. The gonadotropin follicle-stimulating hormone (FSH) and luteinizing hormone (LH) are produced from the anterior pituitary gland. The secretion of gonadotropins also depends on the hypothalamus. Ongoing changes in the concentration of the gonadotropins and ovarian hormones occur during the monthly menstrual cycle (Figure 38-1). Under the influence of FSH and LH, estrogen and progesterone are steroid hormones produced by the ovaries during the menstrual cycle. During the reproductive cycle, the purpose of estrogen and progesterone is to prepare the uterus for implantation of the egg.
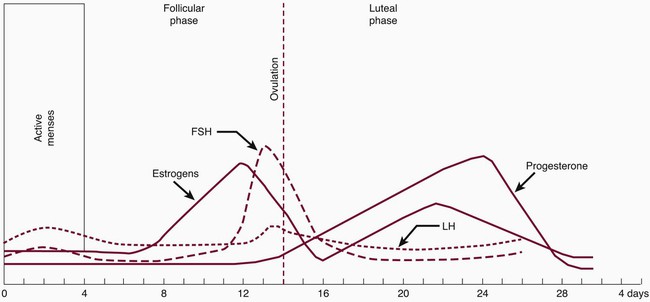
The second phase is called the luteal phase. The developing corpus luteum synthesizes both estradiol and progesterone. Estrogen peaks at 0.2 ng/mL and progesterone at 10.0 ng/mL to complete the rebuilding of the endometrium for implantation of the fertilized egg. The corpus luteum involutes, ovarian hormone levels drop, and menstruation ensues. It has been postulated that ovarian hormones may increase inflammation in gingival tissues and exaggerate the response to local irritants. Gingival inflammation seems to be aggravated by an imbalance or increase in sex hormones. Numerous studies have demonstrated in vitro and in vivo that sex hormones affect and modify the actions of cells of the immune system. In addition, evidence suggests that the interaction between estrogen and cells of the immune system can have nonimmune regulatory effects.7,20 Possible mechanisms have been suggested for the increase in hormonal gingival interaction in the menstrual cycle. Tumor necrosis factor alpha (TNF-α), which fluctuates during the menstrual cycle13; elevated prostaglandin E2 (PGE2) synthesis77; and angiogenetic factors, endothelial growth factors, and receptors may be modulated by progesterone and estrogen, contributing to increases in gingival inflammation during certain stages of the menstrual cycle.
Progesterone has been associated with increased permeability of the microvasculature, altering the rate and pattern of collagen production in the gingiva,71 increasing folate metabolism,96,126 and altering the immune response. During menses, progesterone increases from the second week, peaks at approximately 10 days, and dramatically drops before menstruation. (Note that this is based on a 28-day cycle; individual cycles are variable.) Progesterone plays a role in stimulating the production of prostaglandins that mediate the body’s response to inflammation. PGE2 is one of the major secretory products of monocytes and is increased in inflamed gingiva. Miyagi et al78 found that the chemotaxis of polymorphonuclear leukocytes (PMNs, neutrophils) was enhanced by progesterone but reduced by estradiol. Testosterone did not have a measurable effect on PMN chemotaxis. The researchers suggested that the altered PMN chemotaxis associated with gingival inflammation may be caused by the effects of sex hormones. Physiologic, experimental, and clinical data confirm differences in immune responses between the two sexes.135
Gingival tissues have been reported to be more edematous during menses and erythematous before the onset of menses in some women. A recent study reported higher gingival indices during ovulation and before menstruation despite reported increases in oral symptoms during menses.72 In addition, an increase of gingival exudate has been observed during the menstrual period and is sometimes associated with a minor increase in tooth mobility.39 The incidence of postextraction osteitis is also higher during initiation of menses. No significant hematologic laboratory findings accompany this, other than a slightly reduced platelet count and a slight increase in clotting time.
When the progesterone level is highest (during luteal phase of cycle), intraoral recurrent aphthous ulcers,33 herpes labialis lesions, and candidal infections occur in some women as a cyclic pattern. Because the esophageal sphincter is relaxed by progesterone, women may be more susceptible to gastroesophageal reflux disease (GERD) during this time of the cycle as well. Symptoms of GERD include heartburn, regurgitation, and chest pain, and when reflux is severe, some patients develop unexplained coughing, hoarseness, sore throat, gingivitis, and asthma.109
Management
During PMS, many women exhibit physical symptoms that include fatigue, sweet and salty food cravings, abdominal bloating, swollen hands or feet, headaches, breast tenderness, nausea, and gastrointestinal upset. GERD may make it more uncomfortable for the patient to lay fully supine, especially after a meal, and the woman may have a more sensitive gag reflex. The clinician should be aware that nonsteroidal antiinflammatory drugs (NSAIDs), infection, and acidic foods exacerbate GERD. Patients taking over-the-counter antacids, H2-receptor antagonists (cimetidine, famotidine, nizatidine, ranitidine), prokinetic agents (cisapride, metoclopramide), and proton pump inhibitors (lansoprazole, omeprazole, pantoprazole, or rabeprazole)110 may be GERD patients. These medications interact with some antibiotics and antifungals, and thus a review of their pharmacology is necessary. Fluoride rinses and trays, frequent periodontal debridement, and avoidance of mouthwashes with high alcohol content may reduce the associated gingival and caries sequelae.
PMS is often treated by antidepressants. Selective serotonin reuptake inhibitors (SSRIs) are generally the first-line choice because they have fewer side effects than other antidepressants, do not require blood monitoring, and are safe in overdoses. Women with PMS taking the SSRI fluoxetine were reported to have a 70% response rate. Fluoxetine was ranked the fifth most dispensed prescription (new and refills) in the United States in 1998, but when the patent was lifted, its sales slowed. However, overall SSRIs ranked second in total dollar sales in the 2000s. (Sertraline was ranked twelfth and is the drug of choice for treatment of PMS.143) The clinician should be aware that patients taking fluoxetine have increased side effects with highly protein-bound drugs (e.g., aspirin), and the half-life of diazepam and other central nervous system (CNS) depressants is increased. Additional common SSRIs are fluvoxamine, paroxetine, and citalopram. Other antidepressants that may be prescribed are the selective serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclics, trazodone, mirtazapine, nefazodone, and maprotiline.
Pregnancy
Periodontal Manifestations
The link between pregnancy and periodontal inflammation has been known for many years. In 1778, Vermeeren discussed “toothpains” in pregnancy. In 1818, Pitcarin102 described gingival hyperplasia in pregnancy. Despite awareness regarding pregnancy and its effect on periodontal disease, only recently has evidence indicated an inverse relationship to systemic health. Current research implies periodontal disease may alter the systemic health of the patient and adversely affect the well-being of the fetus by elevating the risk for low-birth-weight, preterm infants.
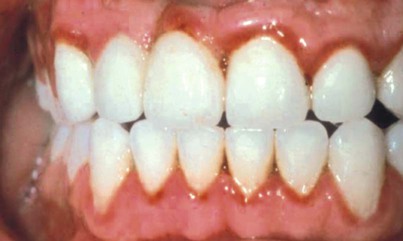
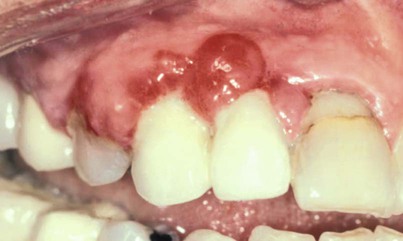
In 1877, Pinard101 recorded the first case of “pregnancy gingivitis.” Only recently has periodontal research begun to focus on causative mechanisms. The occurrence of pregnancy gingivitis is extremely common, occurring in 30% to 100% of all pregnant women.45,62,66,114 It is characterized by erythema, edema, hyperplasia, and increased bleeding. Histologically, the description is the same as for gingivitis. However, the etiologic factors are different despite clinical and histologic similarities. Cases range from mild-to-severe inflammation (Figure 38-2), which can progress to severe hyperplasia, pain, and bleeding (Figures 38-3 and 38-4).
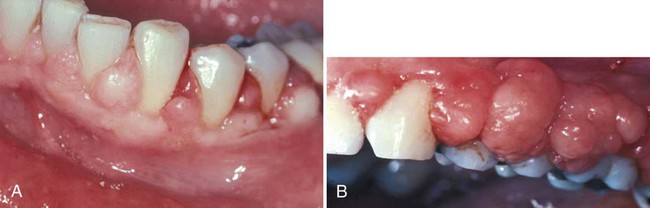
Other growths that resemble pregnancy granulomas must be ruled out, such as central giant cell granulomas or underlying systemic diseases. Periodontal status before pregnancy may influence the progression or severity as the circulating hormones fluctuate. The anterior region of the mouth is affected more often, and interproximal sites tend to be most involved.25 Increased tissue edema may lead to increased pocket depths and may be associated with transient tooth mobility.103 Anterior site inflammation may be exacerbated by increased mouth breathing, primarily in the third trimester, from pregnancy rhinitis. The gingiva is the most common site involved (approximately 70% of all cases), followed by the tongue and lips, buccal mucosa, and palate.114 An increase in attachment loss may represent active periodontal infection accelerated by pregnancy.61
Pyogenic granulomas (“pregnancy tumors,” pregnancy epulis) occur in 0.2% to 9.6% of pregnancies. They are clinically and histologically indistinguishable from pyogenic granulomas occurring in nonpregnant women or in men. Pyogenic granulomas appear most often during the second or third month of pregnancy. Clinically, they bleed easily and become hyperplastic and nodular. When excised, the lesions usually do not leave a large defect. They may be sessile or pedunculated and ulcerated, ranging in color from purplish red to deep blue, depending on the vascularity of the lesion and degree of venous stasis.10 The lesion classically occurs in an area of gingivitis and is associated with poor oral hygiene and calculus. Alveolar bone loss is usually not associated with pyogenic granulomas of pregnancy.
Role of Pregnancy Hormones
Subgingival Plaque Composition.
Epidemiologic studies indicate a relationship between the level of home care and the severity of gingival inflammation. It appears that the association between signs of gingival inflammation and the amount of plaque is greater after parturition than during pregnancy. An alteration in the compositions of subgingival plaque occurs during pregnancy. As mentioned, Kornman and Loesche58 found that during the second trimester, gingivitis and gingival bleeding increased, without an increase in plaque levels. Bacterial anaerobic/aerobic ratios increased, in addition to proportions of Bacteroides melaninogenicus and P. intermedia (2.2% to 10.1%). The authors suggested that estradiol or progesterone can substitute for menadione (vitamin K) as an essential growth factor for P. intermedia but not Porphyromonas gingivalis or Eikenella corrodens. There was also an increase in P. gingivalis during the twenty-first through twenty-seventh weeks of gestation, but this was not statistically significant. The relative increase in the numbers of P. intermedia may be a more sensitive indicator of an altered systemic hormonal situation than clinical parameters of gingivitis.119 A recent study concluded subgingival levels of bacteria associated with periodontitis did not change. P. gingivalis and Tannerella forsythia counts were higher and associated with bleeding on probing at week 12.2
Periodontal Disease and Preterm, Low-Birth-Weight Infant.
Several systematic reviews indicate periodontal disease115,130,137,139 may adversely affect pregnancy outcomes. Intervention trials have shown a positive effect with periodontal therapy and reduction of adverse pregnancy outcomes,50,68,69,91,124 but three large multicenter trials in the United States did not support these results.76,88,120 Studies do indicate that routine nonsurgical periodontal therapy after the first trimester is not associated with adverse pregnancy outcomes.76
Initially, Offenbacher et al90 provided evidence that untreated periodontal disease in pregnant women may be a significant risk factor for preterm (<37 weeks’ gestation), low-birth-weight (<2500 g) infants. The relationship between genitourinary tract infection and preterm, low-birth-weight (PLBW) infants is well documented in human and animal studies. Periodontal researchers, suspecting periodontal disease as another source of infection, found that otherwise low-risk mothers of PLBW infants had significantly more periodontal attachment loss than control mothers having normal-weight infants at birth.
The current opinion is that the correlation of periodontal disease to PLBW births may occur as a result of infection and is mediated indirectly, principally by the translocation of bacterial products such as endotoxin (lipopolysaccharide [LPS]) and the action of maternally produced inflammatory mediators.36 Jared et al48 noted in utero fetal exposure to oral pathogens increases the risk of neonatal intensive care unit admission and length of stay. Biologically active molecules, such as PGE2 and TNF-α, which are normally involved in normal parturition, are raised to artificially high levels by the infection process, which may foster premature labor.3 Gram-negative bacteria in periodontal diseases therefore may permit selective overgrowth or invasion of gram-negative bacteria within the genitourinary tract. Han et al44 documented hematogenous spread of oral bacteria to the amnion, while Madianos et al73 showed that oral bacteria crosses the placental barrier and triggered an immune response by the fetus.
Gingival crevicular fluid (GCF) levels of PGE2 were positively associated with intraamniotic PGE2 levels (p = 0.018), suggesting that gram-negative periodontal infection may present a systemic challenge sufficient to initiate the onset of premature labor as a source of LPS, or through stimulation of secondary inflammatory mediators such as PGE2 and interleukin-1 beta (IL-1β).21 Offenbacher et al89 suggested a dose-response relationship for increasing GCF PGE2 as a marker of current periodontal disease activity and decreasing birth weight. Four organisms associated with mature plaque and progressing periodontitis—T. forsythia, P. gingivalis, A. actinomycetemcomitans, and Treponema denticola—were detected at higher levels in PLBW mothers compared with normal-birth-weight controls (see Chapter 28). Despite research supporting the association of periodontal disease and PLBW,22,23 more studies with improved methodology are needed to assess the validity of the association.
Preeclampsia.
A systematic review of preeclampsia and periodontitis indicated an increased risk during pregnancy. Preeclampsia is a life-threatening condition in late pregnancy characterized by high blood pressure and excess urine protein. High C-reactive protein levels also are associated with preeclampsia in this population.112,117,129
Maternal Immunoresponse
The maternal immune system is thought to be suppressed during pregnancy. This response may allow the fetus to survive as an allograft. Documentation of immunosuppressive factors in the sera of pregnant women can be noted by marked increase of monocytes (which in large numbers inhibit in vitro proliferative responses to mitogens, allogenic cells, and soluble antigen),128 and pregnancy-specific βl-glycoproteins contribute to diminished lymphocyte responsiveness to mitogens and antigens.11 In addition, a decrease in the ratio of peripheral T helper cells to T suppressor cells (CD4/CD8) has been reported to occur throughout pregnancy.105 These changes in maternal immunoresponsiveness suggest an increased susceptibility to developing gingival inflammation. In one study, the gingival index was higher, but percentages of T3, T4, and B cells appeared to decrease in peripheral blood and gingival tissues during pregnancy compared with a control group.1 Other studies report decreased PMN (neutrophil) chemotaxis, depression of cell-mediated immunity, phagocytosis, and a decreased T-cell response with elevated ovarian hormone levels, especially progesterone.104 Decreased in vitro responses of peripheral blood lymphocytes to several mitogens and to a preparation of P. intermedia have been reported.12,67,87 Also, evidence suggests a decrease in the absolute numbers of CD4+ cells in peripheral blood during pregnancy compared with the number of these cells postpartum.80,88 Lapp et al60 suggested that high levels of progesterone during pregnancy affect the development of localized inflammation by downregulation of IL-6 production, rendering the gingiva less efficient at resisting the inflammatory challenges produced by the bacteria. A recent study indicated live preterm birth is associated with decreased levels of immunoglobulin G (IgG) antibody to periodontal pathogens in women with periodontitis when assessed during the second trimester but not associated with birth outcomes.27
Also, ovarian hormone stimulates the production of prostaglandins, in particular PGE1 and PGE2, which are potent mediators of the inflammatory response. With the prostaglandin acting as an immunosuppressant, gingival inflammation may increase when the mediator level is high.30,93 Kinnby et al55 found that high progesterone levels during pregnancy influenced plasminogen activator inhibitor type 2 (PAI-2) and disturbed the balance of the fibrinolytic system. Because PAI-2 serves as an important inhibitor of tissue proteolysis, this research implies that components of the fibrinolytic system may be involved in the development of pregnancy gingivitis.
During pregnancy, sex hormonal levels rise dramatically (Box 38-1). Progesterone reaches levels of 100 ng/mL, 10 times the peak luteal phase of menses. Estradiol in the plasma may reach 30 times higher levels than during the reproductive cycle. In early pregnancy and during the normal ovarian cycle, the corpus luteum is the major source of estrogen and progesterone. During pregnancy the placenta begins to produce estrogens and progesterone.
Estrogen may regulate cellular proliferation, differentiation, and keratinization, whereas progesterone influences the permeability of the microvasculature,63,64 alters the rate and pattern of collagen production, and increases the metabolic breakdown of folate (necessary for tissue maintenance).141 High concentration of sex hormones in gingival tissues, saliva, serum, and GCF also may exaggerate the response.
Regulation of most cellular processes by hormones occurs through the interaction of these products with intracellular receptors. The resulting effects depend on the concentration of unbound hormone diffused through the cell membrane. Vittek et al132 demonstrated specific estrogen and progesterone receptors in gingival tissues, providing direct biochemical evidence that this tissue may function as a target organ for sex hormones. Muramatsu and Takaesu83 found increasing concentration of sex hormones in saliva from the first month and peaking in the ninth month of gestation, along with increasing percentages of P. intermedia. Probing depth, number of gingival sites with bleeding, and redness increased until 1 month postpartum. Also, evidence indicates sex hormone concentration in GCF, providing a growth media for periodontal pathogens.
Other Oral Manifestations of Pregnancy
Xerostomia is a frequent complaint among pregnant women. One study found this persistent dryness in 44% of pregnant participants.28
A rare finding in pregnancy is ptyalism, or sialorrhea. This excessive secretion of saliva usually begins at 2 to 3 weeks of gestation and may abate at the end of the first trimester. The etiology of ptyalism has not been identified, but it may result from the inability of nauseated gravid women to swallow normal amounts of saliva, rather than from a true increase in saliva production.19
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses