Periodontal Treatment of Medically Compromised Patients
Many patients seeking dental care have significant medical conditions that may alter both the course of their oral disease and the therapy provided. Older periodontal patients will have a greater likelihood of having underlying disease. Therefore the therapeutic responsibility of the clinician includes identification of the patient’s medical problems to formulate proper treatment plans. Thorough medical histories are paramount.1 If significant findings are unveiled, consultation with or referral of the patient to an appropriate physician may be indicated. This ensures correct patient management and provides medicolegal coverage to the clinician.
Cardiovascular Diseases
Cardiovascular diseases are the most prevalent category of systemic disease in the United States and many other countries, and they are more common with increasing age.2 Health histories should be closely scrutinized for cardiovascular problems. These conditions include hypertension, angina pectoris, myocardial infarction (MI), previous cardiac bypass surgery, previous cerebrovascular accident (CVA), congestive heart failure (CHF), presence of cardiac pacemakers or automatic cardioverter-defibrillators, and infective endocarditis (IE).
Hypertension
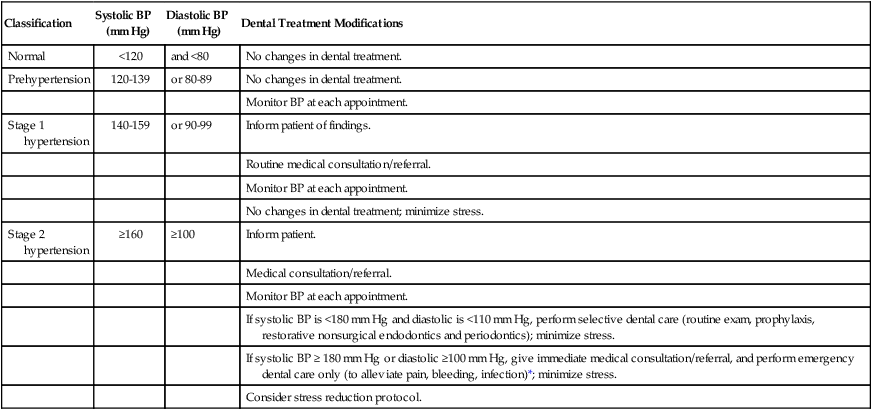
Hypertension, the most common cardiovascular disease, affects more than 50 million American adults, many of whom are undiagnosed.3 In 2003, the National Heart, Lung and Blood Institute issued revised guidelines for evaluation and management of hypertension.4–6 The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7) guidelines4 simplified the classification of blood pressure (Table 37-1).
TABLE 37-1
Classification of Adult Blood Pressure
| Classification | Systolic BP (mm Hg) | Diastolic BP (mm Hg) | Dental Treatment Modifications |
| Normal | <120 | and <80 | No changes in dental treatment. |
| Prehypertension | 120-139 | or 80-89 | No changes in dental treatment. |
| Monitor BP at each appointment. | |||
| Stage 1 hypertension | 140-159 | or 90-99 | Inform patient of findings. |
| Routine medical consultation/referral. | |||
| Monitor BP at each appointment. | |||
| No changes in dental treatment; minimize stress. | |||
| Stage 2 hypertension | ≥160 | ≥100 | Inform patient. |
| Medical consultation/referral. | |||
| Monitor BP at each appointment. | |||
| If systolic BP is <180 mm Hg and diastolic is <110 mm Hg, perform selective dental care (routine exam, prophylaxis, restorative nonsurgical endodontics and periodontics); minimize stress. | |||
| If systolic BP ≥ 180 mm Hg or diastolic ≥100 mm Hg, give immediate medical consultation/referral, and perform emergency dental care only (to alleviate pain, bleeding, infection)*; minimize stress. | |||
| Consider stress reduction protocol. |
*Risk of providing emergency dental care must outweigh risk of possible hypertensive complications.4–6
Compared with previous classification7,8 schemes that focused on diastolic blood pressure (BP), the JNC-7 guidelines4 emphasize the importance of systolic BP greater than 140 mm Hg. Systolic blood pressure greater than 140 mm Hg is considered a greater risk factor for cardiovascular disease than elevated diastolic pressure. JNC-7 also introduced a category known as prehypertension to replace the more innocuous terms “high normal” and “borderline” hypertension. People with systolic BP between 120 and 139 mm Hg or diastolic BP between 80 and 89 mm Hg are classified as “prehypertensive.” Hypertension is now classified into only two categories versus three under past classification schemes for simplicity and because treatment for categories 2 and 3 was essentially the same. Stage 1 hypertension is defined by systolic pressure of 140 to 159 mm Hg or diastolic pressure of 90 to 99 mm Hg. Stage 2 hypertension is defined by a systolic pressure greater than 160 mm Hg or diastolic pressure greater than 100 mm Hg.
Hypertension is not diagnosed on a single elevated BP recording. Rather, classification is usually based on the average value of two or more BP readings taken at two or more appointments. The higher value of either the systolic or diastolic pressure determines the patient’s classification. Patients with hypertension enter the dental practice every day and are particularly common among the older population seen in most periodontal practices. Evidence from the Framingham Heart Study revealed that people with normal BP at age 55 still have a 90% risk of becoming hypertensive later in life.9
In early hypertension, the patient may be asymptomatic. If not identified and diagnosed, hypertension may persist and increase in severity, leading eventually to coronary artery disease, angina, MI, CHF, CVA, or kidney failure.10 The dental office can play a vital role in the detection of hypertension and maintenance care of the patient with hypertensive disease. The first dental office visit should include two BP readings spaced at least 10 minutes apart, which are averaged and used as a baseline. Before the clinician refers a patient to a physician because of elevated BP, readings should be taken at a minimum of two appointments, unless the measurements are extremely high (i.e., systolic pressure >180 mm Hg or diastolic pressure >100 mm Hg). The periodontal recall system is an ideal method for hypertension detection and monitoring. Almost three of every four adult patients with hypertension in the United States do not control their BP well enough to attain the goal of systolic pressure less than 140 mm Hg and diastolic pressure less than 90 mm Hg.11 Lack of compliance with antihypertensive therapy is the primary reason for this failure. Dentists can help achieve greater success in managing hypertension by taking BP readings at each periodontal recall visit.
Periodontal procedures should not be performed until accurate BP measurements and histories have been taken to identify those patients with significant hypertensive disease. The time of day also should be recorded because BP varies significantly throughout the day.12 Table 37-1 outlines appropriate medical referral or consultation and dental treatment modifications, depending on the patient’s stage of hypertension.
Dental treatment for hypertensive patients is generally safe as long as stress is minimized.10,13 If a patient is currently receiving antihypertensive therapy, consultation with the physician may be warranted regarding the current medical status, medications, periodontal treatment plan, and patient management. Many physicians are not knowledgeable about the nature of specific periodontal procedures. The dentist must inform the physician regarding the estimated degree of stress, length of the procedures, and complexity of the individualized treatment plan. Morning dental appointments were once suggested for hypertensive patients. However, recent evidence indicates that BP generally increases around awakening and peaks at midmorning.12,14,15 Lower BP levels occur in the afternoon; therefore afternoon dental appointments may be preferred.
When treating hypertensive patients, the clinician should not use a local anesthetic containing an epinephrine concentration greater than 1 : 100,000 nor should a vasopressor be used to control local bleeding. Local anesthesia without epinephrine may be used for short procedures (<30 minutes). In a patient with hypertensive disease, however, it is important to minimize pain by providing profound local anesthesia to avoid an increase in endogenous epinephrine secretion.10,13
The benefits of the small doses of epinephrine used in dentistry far outweigh the potential for hemodynamic compromise. The smallest possible dose of epinephrine should be used, and aspiration before injection of local anesthetics is critical. Intraligamentary injection is generally contraindicated because hemodynamic changes are similar to intravascular injection.16 If the hypertensive patient exhibits anxiety, use of conscious sedation in conjunction with periodontal procedures may be warranted17 (see Chapter 36).
Beta-adrenergic receptor antagonists, or β-blockers, are typically used to treat hypertension (Table 37-2). β-blockers are either cardioselective, blocking only β-1 cardiac receptors (β1 receptors), or nonselective, blocking both β-1 cardiac receptors and β-2 peripheral receptors (β2 receptors). Epinephrine, an α-adrenergic and β-adrenergic agonist, produces an increase in heart rate through direct stimulation of cardiac β-1 receptors. Epinephrine also stimulates α-adrenergic receptors, producing vasoconstriction of arteries, as well as β-2 receptors, causing vasodilation of skeletal muscle arterioles. Administration of local anesthetics containing epinephrine to patients taking nonselective β-blockers (e.g., propranolol, nadolol) may cause elevated BP.18 Epinephrine-induced α-adrenergic stimulation results in vasoconstriction and increased BP. Because the patient’s nonselective medication has blocked the β-2 receptors, epinephrine will not stimulate the normal compensatory β-2 receptor–induced vasodilation. This may result in dramatically increased BP, followed by reflex bradycardia mediated by the vagus nerve and carotid baroreceptors. The end result is a patient with severe hypertension and bradycardia, resulting in a dangerous decrease in vascular perfusion and possible death. Because of this potential complication, epinephrine-containing local anesthetics should be used cautiously and only in very small amounts in patients taking nonselective β-blockers, with careful monitoring of vital signs.10,18
TABLE 37-2
Nonselective and Selective β-Adrenergic Receptor Antagonists (β-Blockers)
| Generic Name | Trade Name |
| Nonselective β-Blockers | |
| Carvedilol | Coreg |
| Carteolol hydrochloride | Cartrol |
| Nadolol | Corgard |
| Penbutolol sulfate | Levatol |
| Pindolol | Visken |
| Propranolol hydrochloride | Inderal; Inderal LA |
| Timolol maleate | Blocadren |
| Selective β-Blockers | |
| Acebutolol hydrochloride | Sectral |
| Atenolol | Tenormin |
| Betaxolol hydrochloride | Kerlone |
| Bisoprolol fumarate | Zebeta |
| Metoprolol tartrate | Lopressor |
| Metoprolol succinate | Toprol-XL |
The clinician should be aware of the many side effects of various antihypertensive medications. Postural hypotension is common and can be minimized by slow positional changes in the dental chair.10 Depression is a side effect of which many patients are unaware. Nausea, sedation, oral dryness, lichenoid drug reactions, and gingival overgrowth are associated with certain classes of antihypertensive agents.13
Ischemic Heart Diseases
Ischemic heart disease (Figure 37-1) includes disorders such as angina pectoris and myocardial infarction. Angina pectoris occurs when myocardial oxygen demand exceeds supply, resulting in temporary myocardial ischemia.5 Patients with a history of unstable angina pectoris (angina that occurs irregularly or on multiple occasions without predisposing factors) should be treated only for emergencies and then in consultation with their physician. Patients with stable angina (angina that occurs infrequently, is associated with exertion or stress, and is easily controlled with medication and rest) can undergo elective dental procedures. Because stress often induces an acute anginal attack, stress reduction is important. Profound local anesthesia is vital, and conscious sedation may be indicated for anxious patients17 (see Chapter 36). Supplemental oxygen delivered by nasal cannula may also help prevent intraoperative anginal attacks.
Patients who treat acute anginal attacks with nitroglycerin should be instructed to bring their medication to dental appointments. Nitroglycerin should also be kept in the office emergency medical kit. For particularly stressful procedures, the patient may take a nitroglycerin tablet preoperatively to prevent angina, although this generally is not necessary. The patient’s nitroglycerin should be readily accessible on the dental tray in case it is needed during treatment. Because the shelf life of nitroglycerin is relatively short, the expiration date of the patient’s nitroglycerin should be noted, as should the expiration date of the nitroglycerin in the office’s emergency medical kit. Also, patients with angina may be taking longer-acting forms of nitroglycerin (tablet, patch), β-blockers, or calcium channel blockers (also used in treatment of hypertension) for prevention of angina. Restrictions on use of local anesthetics containing epinephrine are similar to those for the patient with hypertension. In addition, intraosseous injection with epinephrine-containing local anesthetics using special systems (e.g., Stabident, Fairfax Dental) should be done cautiously in patients with ischemic heart disease, because it results in transient increases in heart rate and myocardial oxygen demand.19
1. Discontinue the periodontal procedure.
2. Administer 1 tablet (0.3 to 0.6 mg) of nitroglycerin sublingually.
3. Reassure the patient, and loosen restrictive garments.
4. Administer oxygen with the patient in a reclined position.
5. If the signs and symptoms cease within 3 minutes, complete the periodontal procedure if possible, making sure that the patient is comfortable. Terminate the procedure at the earliest convenient time.
6. If the anginal signs and symptoms do not resolve with this treatment within 2 to 3 minutes, administer another dose of nitroglycerin, monitor the patient’s vital signs, call the patient’s physician, and be ready to accompany the patient to the emergency department.
7. A third nitroglycerin tablet may be given 3 minutes after the second. Chest pain that is not relieved by three tablets of nitroglycerin indicates likely myocardial infarction. The patient should be transported to the nearest emergency medical facility immediately.
In recent years, nitroglycerin lingual spray formulations have been popular in hospital pharmacies because of the increased shelf life as compared to nitroglycerin tablets.20 The lingual spray has been reported to provide a greater and more rapid vasodilation with a longer duration of action.21,22 The convenience and advantages of a nitroglycerin lingual spray are appealing, but the accuracy of dose delivery has been questioned and warrants additional studies before it can be recommended to replace the known tablet regimen.20
Myocardial infarction (MI) is the other category of ischemic heart disease encountered in dental practice. Dental treatment is generally deferred for at least 6 months after MI because peak mortality occurs during this time.23 After 6 months, MI patients can usually be treated using techniques similar to those for the stable angina patient.
Congestive Heart Failure
Congestive heart failure (CHF) is a condition in which the pump function of the heart is unable to supply sufficient amounts of oxygenated blood to meet the body’s needs.23 CHF usually begins with left ventricular failure caused by a disproportion between the hemodynamic load and the capacity to handle that load. CHF may be caused by a chronic increase in workload (as in hypertension or aortic, mitral, pulmonary, or tricuspid valvular disease), direct damage to the myocardium (as in MI or rheumatic fever), or an increase in the body’s oxygen requirements (as in anemia, thyrotoxicosis, or pregnancy).
Patients with poorly controlled or untreated CHF are not candidates for elective dental procedures. These individuals are at risk for sudden death, usually from ventricular arrhythmias.24 For patients with treated CHF, the clinician should consult with the physician regarding the severity of CHF, underlying etiology, and current medical management. Medical management of CHF may include use of calcium channel blockers, direct vasodilators, diuretics, angiotensin-converting enzyme (ACE) inhibitors, α-receptor blockers, and cardiotonic agents such as digoxin.25,26 Each of these medications has potential side effects that may have an impact on periodontal therapy. Because of the presence of orthopnea (inability to breathe unless in an upright position) in some CHF patients, the dental chair should be adjusted to a comfortable level for the patient rather than being placed in a supine position. Short appointments, stress reduction with profound local anesthesia and possibly conscious sedation, and use of supplemental oxygen should be considered.10,23
Cardiac Pacemakers and Implantable Cardioverter-Defibrillators
Cardiac arrhythmias are most often treated with medications; however, some are also treated with implantable pacemakers or automatic cardioverter-defibrillators.10,24,27 Pacemakers are usually implanted in the chest wall and enter the heart transvenously. Automatic cardioverter-defibrillators are more often implanted subcutaneously near the umbilicus and have electrodes passing into the heart transvenously or directly attached to the epicardium. Consultation with the patient’s physician allows determination of the underlying cardiac status, the type of pacemaker or automatic cardioverter-defibrillator, and any precautionary measures to be taken. Older pacemakers were unipolar and could be disrupted by dental equipment that generated electromagnetic fields, such as ultrasonic and electrocautery units. Newer units are bipolar and are generally not affected by dental equipment. Automatic cardioverter-defibrillators activate without warning when certain arrhythmias occur. This may endanger the patient during dental treatment because such activation often causes sudden patient movement. Stabilization of the operating field during periodontal treatment with bite blocks or other devices can prevent unexpected trauma.
Infective Endocarditis
Infective endocarditis (IE) is a disease in which microorganisms colonize the damaged endocardium or heart valves.28 Although the incidence of IE is low, it is a serious disease with a poor prognosis, despite modern therapy. The term infective endocarditis is preferred to the previous term bacterial endocarditis because the disease can also be caused by fungi and viruses. The organisms most often encountered in IE are α-hemolytic streptococci (e.g., Streptococcus viridans). However, nonstreptococcal organisms often found in the periodontal pocket have been increasingly implicated, including Eikenella corrodens, Aggregatibacter actinomycetemcomitans, Capnocytophaga, and Lactobacillus species.29
Since the last American Heart Association (AHA) publication on prevention of IE in 1997,30 many have questioned the efficacy of antimicrobial prophylaxis to prevent IE in patients who undergo dental or other procedures and have suggested that the AHA guidelines should be revised.31,32 Members of the Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the AHA Council on Cardiovascular Disease in the Young, and a national and international group of experts on IE extensively reviewed data published on the prevention of IE. The committee concluded that only an extremely small number of IE cases might be prevented by antibiotic prophylaxis for dental procedures (even if such therapy was 100% effective). Consequently, the guidelines were changed and published in a 2008 report.33 The new guidelines advise that IE prophylaxis should only be recommended for cardiac conditions with the highest risk of adverse outcome from IE (Box 37-1). For these patients, antibiotic prophylaxis is recommended for all dental procedures that involve manipulation of the gingival tissues, periapical tissues, or perforation of the oral mucosa. Antibiotic prophylaxis is not indicated for individuals on the basis of an increased lifetime risk of contracting IE.
The practice of periodontics is intimately concerned with the prevention of IE. However, bacteremia may occur even in the absence of dental procedures, especially in individuals with poor oral hygiene and significant periodontal inflammation. In fact, IE is much more likely to result from frequent exposure to random bacteremias associated with daily activities than caused by a dental procedure.33 Thus the prevention of periodontal inflammation is paramount. The AHA states that patients who are at risk for IE should “establish and maintain the best possible oral health to reduce potential sources of bacterial seeding.” To provide adequate preventive measures for IE, the clinician’s major concern should be to reduce the microbial population in the oral cavity so as to minimize soft tissue inflammation and bacteremia.
Preventive measures to reduce the risk of IE should consist of the following:
1. Define the susceptible patient. A careful medical history will disclose the previously mentioned susceptible patients. Health questioning should cover history of all potential categories of risk. If any doubt exists, the patient’s physician should be consulted.
2. Provide oral hygiene instruction. Oral hygiene should be practiced with methods that improve gingival health. In patients with significant gingival inflammation, oral hygiene should initially be limited to gentle procedures (i.e., oral rinses and gentle toothbrushing with a soft brush) to minimize bleeding. As gingival health improves, more aggressive oral hygiene may be initiated. Oral irrigators are generally not recommended because their use may induce bacteremia.34 Susceptible patients should be encouraged to maintain the highest level of oral hygiene once soft tissue inflammation is controlled.
3. During periodontal treatment, currently recommended antibiotic prophylactic regimens (Table 37-3) should be practiced with all high-risk patients. If any doubt regarding susceptibility exists, the patient’s physician should be consulted. In patients who have been receiving continuous oral penicillin for secondary prevention of rheumatic fever, penicillin-resistant α-hemolytic streptococci are occasionally found in the oral cavity. It therefore is recommended that an alternate regimen be followed instead. Likewise, if the periodontal patient is taking a systemic antibiotic as part of periodontal therapy, changes in the IE prophylaxis regimen may be indicated. For example, a patient currently taking a penicillin agent after regenerative therapy may be placed on azithromycin before the next periodontal procedure. Patients with early-onset forms of periodontitis often have high levels of A. actinomycetemcomitans in the subgingival plaque. This organism has been associated with IE and is often resistant to penicillins. Therefore, in patients with aggressive periodontitis who should be given prophylaxis, Slots et al35 suggested using tetracycline, 250 mg, four times daily for 14 days to eliminate or reduce A. actinomycetemcomitans, followed by the conventional prophylaxis protocol at the time of dental treatment.
TABLE 37-3
Recommended Antibiotic Prophylaxis Regimens for Periodontal Procedures in Adults at Risk for Infective Endocarditis
| Regimen | Antibiotic | Dosage* |
| Standard oral regimen | Amoxicillin | 2.0 g |
| 30-60 minutes before procedure | ||
| Alternate regimen for patients allergic to amoxicillin/penicillin | Clindamycin | 600 mg 30-60 minutes before procedure |
| or | ||
| Azithromycin or clarithromycin | 500 mg 30-60 minutes before procedure | |
| or | ||
| Cephalexin or cefadroxil* | 2.0 g 30-60 minutes before procedure | |
| Patients unable to take oral medications | Ampicillin | 2.0 g intramuscularly or intravenously within 30 minutes before procedure |
| Patients unable to take oral medications and allergic to penicillin | Clindamycin | 600 mg intravenously within 30 minutes before procedure (must be diluted and injected slowly) |
| or | ||
| Cefazolin† | 1.0 g intramuscularly or intravenously within 30 minutes before procedure |
*Cephalosporins should not be used in patients with immediate-type hypersensitivity reactions to penicillins (e.g., urticaria, angioedema, anaphylaxis).
4. Periodontal treatment should be designed for susceptible patients to accommodate their particular degree of periodontal involvement. The nature of periodontal therapy enhances the problems related to the prophylaxis of subacute IE. Patients are faced with long-term therapy, healing periods that extend beyond a 1-day antibiotic regimen, multiple visits, and procedures that easily elicit gingival bleeding.
• Periodontal disease is an infection with potentially wide-ranging systemic effects. In patients at risk for IE, every effort should be made to eliminate this infection. Teeth with severe periodontitis and a poor prognosis may require extraction. Teeth with less severe involvement in a motivated patient should be retained, treated, and maintained closely.
• All periodontal treatment procedures (including probing) require antibiotic prophylaxis; gentle oral hygiene methods are excluded. Pretreatment chlorhexidine rinses are recommended before all procedures, including periodontal probing, because these oral rinses significantly reduce the presence of bacteria on mucosal surfaces.30
• To reduce the number of visits required and thereby minimize the risk of developing resistant bacteria, numerous procedures may be accomplished at each appointment, depending on the patient’s needs and ability to tolerate dental treatment.10
• When possible, allow at least 7 days between appointments (preferably 10 to 14 days). If this is not possible, select an alternative antibiotic regimen for appointments within a 7-day period.
• Evidence does not support or refute a need to place patients at risk for IE on extended antibiotic regimens after treatment.10 Therefore patients who have had periodontal surgery are not generally placed on antibiotics for the first week of healing (unless there are specific indications to do so). If patients are placed on such regimens, the dosages are inadequate to prevent endocarditis during ensuing appointments. Therefore the standard prophylactic antibiotic dose is still needed. For example, if a patient was placed on 250 mg of amoxicillin three times a day for 10 days after periodontal surgery and was returning to the office for more treatment on the seventh day, the patient would still require a full 2.0-g dose of amoxicillin before that treatment. Alternatively, clindamycin or azithromycin could be used at the second appointment.
• Regular recall appointments, with an emphasis on oral hygiene reinforcement and maintenance of periodontal health, are extremely important for patients susceptible to IE.
Cerebrovascular Accident
To prevent a repeat stroke, active infections should be treated aggressively because even minor infection may alter blood coagulation and trigger thrombus formation and ensuing cerebral infarction. The clinician should counsel the patient about the importance of thorough oral hygiene.36 Poststroke weakness of the facial area or paralysis of extremities may make oral hygiene procedures extremely difficult.37 The clinician may need to modify oral hygiene instruments for ease of use, perhaps in consultation with an occupational therapist. Long-term chlorhexidine rinses may greatly aid in plaque control.
Dental clinicians should treat post-CVA patients with the following guidelines in mind:
1. No periodontal therapy (unless for an emergency) should be performed for 6 months because of the high risk of recurrence during this period.
2. After 6 months, periodontal therapy may be performed using short appointments with an emphasis on minimizing stress. Profound local anesthesia should be obtained using the minimal effective dose of local anesthetic agents. Concentrations of epinephrine greater than 1 : 100,000 are contraindicated.
3. Light conscious sedation (inhalation, oral, or parenteral) may be used for anxious patients. Supplemental oxygen is indicated to maintain thorough cerebral oxygenation.
4. Stroke patients are frequently placed on oral anticoagulants. Previously, it was thought that for procedures entailing significant bleeding, such as periodontal surgery or tooth extraction, the anticoagulant regimen may need adjustment, depending on the level of anticoagulation at which the patient is maintained. However, recent evidence regarding the risks of altering anticoagulation therapy suggests that it may be more prudent to provide treatment without changing it (see later section on anticoagulant/antiplatelet therapy). Any changes in anticoagulant therapy regimens for a stroke patient should always be done in consultation with the patient’s physician.
5. BP should be monitored carefully. Recurrence rates for CVAs are high, as are rates of associated functional deficits.
Endocrine Disorders
Diabetes
The diabetic patient requires special precautions before periodontal therapy. The two major types of diabetes are type 1 (formerly known as “insulin-dependent diabetes”) and type 2 (formerly called “non–insulin-dependent diabetes”).38 Over the past decade, the medical management of diabetes has changed significantly in an effort to minimize the debilitating complications associated with this disease.39,40 Patients are more tightly managing their blood glucose levels (glycemia) through diet, oral agents, and insulin therapy.41
If the clinician detects intraoral signs of undiagnosed or poorly controlled diabetes, a thorough history is indicated.42 The classic signs of diabetes include polydipsia (excessive thirst), polyuria (excessive urination), and polyphagia (excessive hunger, often with unexplained concurrent weight loss). If the patient has any of these signs or symptoms, or if the clinician’s index of suspicion is high, further investigation with laboratory studies and physician consultation is indicated. Periodontal therapy has limited success in the presence of undiagnosed or poorly controlled diabetes.
1. Consult the patient’s physician.
2. Analyze laboratory tests (Box 37-2): fasting blood glucose and casual glucose.43
3. Rule out acute orofacial infection or severe dental infection; if present, provide emergency care immediately.
4. Establish best possible oral health through nonsurgical debridement of plaque and calculus; institute oral hygiene instruction. Limit more advanced care until diagnosis has been established and good glycemic control obtained.
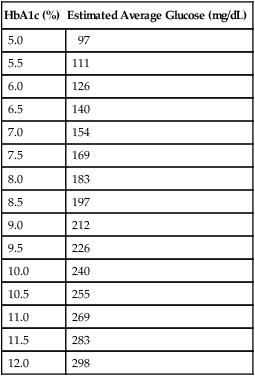
If a patient is known to have diabetes, it is critical that the level of glycemic control be established before initiating periodontal treatment. The fasting glucose and casual glucose tests provide “snapshots” of the blood glucose concentration at the time the blood was drawn; these tests reveal nothing about long-term glycemic control. The primary test used to assess glycemic control in a known diabetic individual is the glycosylated (or glycated) hemoglobin (Hb) assay (Box 37-3). Two different tests are available, the HbA1 and the HbA1c assay; the HbA1c is used more often.41 This assay has been shown by a large international study to provide an accurate measure of the average blood glucose concentrations over the preceding 2 to 3 months.44 Table 37-4 lists the average blood glucose concentrations for HbA1c values from that study, and Figure 37-2 is a simplified graphic representation of the data. The therapeutic goal for many patients is to achieve and maintain an HbA1c below 8%. Patients with relatively well-controlled diabetes (HbA1c < 8%) usually respond to therapy in a manner similar to nondiabetic individuals.45–47 Poorly controlled patients (HbA1c > 10%) often have a poor response to treatment, with more postoperative complications and less favorable long-term results38,46 (see Figure 11-3). Improvements in HBA1c values after periodontal therapy may provide an indication of the potential response.
TABLE 37-4
Comparison of HbA1c Values to Estimated Average Glucose Measurements
| HbA1c (%) | Estimated Average Glucose (mg/dL) |
| 5.0 | 97 |
| 5.5 | 111 |
| 6.0 | 126 |
| 6.5 | 140 |
| 7.0 | 154 |
| 7.5 | 169 |
| 8.0 | 183 |
| 8.5 | 197 |
| 9.0 | 212 |
| 9.5 | 226 |
| 10.0 | 240 |
| 10.5 | 255 |
| 11.0 | 269 |
| 11.5 | 283 |
| 12.0 | 298 |
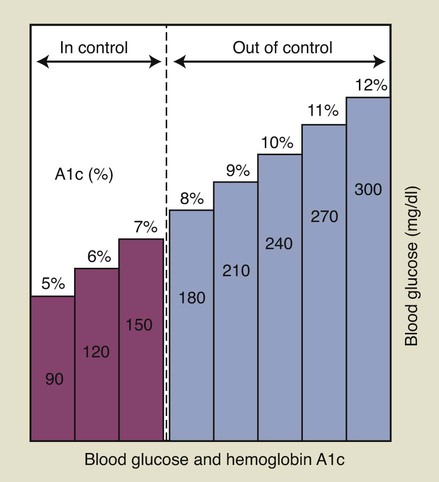
As discussed in Chapter 11, periodontal infection may worsen glycemic control and should be managed aggressively. Diabetic patients with periodontitis should receive oral hygiene instructions, mechanical debridement to remove local factors, and regular maintenance. When possible, an HbA1c of less than 10% should be established before surgical treatment is performed. Systemic antibiotics are not needed routinely, although recent evidence indicates that tetracycline antibiotics in combination with scaling and root planing may positively influence glycemic control. If the patient has poor glycemic control and surgery is absolutely needed, prophylactic antibiotics may be given; penicillins are most often used for this purpose. Frequent reevaluation after active therapy is needed to assess treatment response and prevent recurrence of periodontitis.
1. Patients should be asked to bring their glucometer to the dental office at each appointment.
2. Patients should check their blood glucose before any long procedure to obtain a baseline level. Patients with blood glucose levels at or below the lower end of normal before the procedure may become hypoglycemic intraoperatively. It is advisable to have such a patient consume some carbohydrate before starting treatment. For example, if a 2-hour procedure is planned and the pretreatment glucose level is 70 mg/dL (lower end of normal range), providing 4 oz of juice preoperatively may help prevent hypoglycemia during treatment. If pretreatment glucose levels are excessively high, the clinician should determine whether or not the patient’s glycemic control has been poor recently. This can be done by thorough patient questioning and by determining the most recent HbA1c values. If glycemic control has been poor over the preceding few months, the procedure may need to be postponed until better glycemic control is established. If glycemic control has been good, and the currently high glucometer reading is a fairly isolated event, the surgical procedure may proceed.
3. If the procedure lasts several hours, it is often beneficial to check the glucose level during the procedure to ensure that the patient does not become hypoglycemic.
4. After the procedure, the blood glucose can be checked again to assess fluctuations over time.
5. Any time the patient feels symptoms of hypoglycemia, blood glucose should be checked immediately. This may prevent onset of severe hypoglycemia, a medical emergency.
The most common dental office complication seen in diabetic patients taking insulin is symptomatic low blood glucose, or hypoglycemia (Box 37-4). Hypoglycemia is also associated with the use of numerous oral agents (Table 37-5). In patients receiving conscious sedation, the warning signs of an impending hypoglycemic episode may be masked, making the patient’s glucometer one of the best diagnostic aids. Hypoglycemia does not usually occur until blood glucose levels fall below 60 mg/dL. However, in patients with poor glycemic control who have prolonged hyperglycemia (high blood glucose levels), a rapid drop in glucose can precipitate signs and symptoms of hypoglycemia at levels well above 60 mg/dL.
TABLE 37-5
Oral Agents Used in Management of Diabetes
| Agent | Action | Risk of Hypoglycemia |
| Sulfonylureas (first generation): Chlorpropamide Tolbutamide Tolazamide |
Stimulate pancreatic insulin secretion | ++ |
| Sulfonylureas (second generation): Glyburide Glipizide |
Stimulate pancreatic insulin secretion | +++ |
| Sulfonylureas (third generation): Glimepiride |
Stimulate pancreatic insulin secretion | + |
| Meglitinides: Repaglinide Nateglinide |
Stimulate rapid pancreatic insulin secretion (different mechanism than sulfonylureas) | + |
| Biguanides: Metformin |
Block production of glucose by liver; improve tissue sensitivity to insulin | − |
| Thiazolidinediones: Rosiglitazone Pioglitazone |
Improve tissue sensitivity to insulin | − |
| α-Glucosidase inhibitors: Acarbose Miglitol |
Slow absorption of some carbohydrates from gut, decreasing postprandial peaks in glycemia | − |
| Dipeptidyl peptidase-4 (DPP-4) inhibitors: Sitagliptin Saxagliptin |
Inhibit the enzyme DPP-4; enable the pancreas to produce more insulin but only after food ingestion | − |
| Combination agents | Combine two different oral agents into single drug | Risk depends on which drugs are combined |
As medical management of diabetes has intensified over the last decade, the incidence of severe hypoglycemia has risen.48 The clinician should question diabetic patients about past episodes of hypoglycemia. Hypoglycemia is more common in patients with better glycemic control. When planning dental treatment, it is best to schedule appointments before or after periods of peak insulin activity. This requires knowledge of the pharmacodynamics of the drugs being taken by the diabetic patient. Patients taking insulin are at greatest risk, followed by those taking sulfonylurea agents. Metformin and thiazolidinediones generally do not cause hypoglycemia (see Table 37-5).
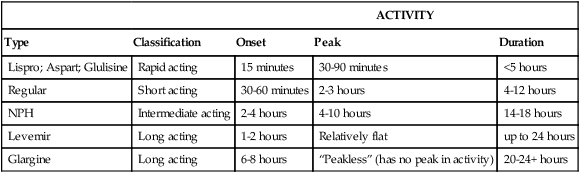
Insulins are classified as rapid-acting, short-acting, intermediate-acting, or long-acting agents (Table 37-6). The categories vary in their onset, peak, and duration of activity. It is important that the clinician establish exactly which insulins the diabetic patient takes, the amount, the number of times per day, and the time of the last dose. Periodontal treatment often can be timed to avoid peak insulin activity. Many diabetic patients take multiple injections each day, in which case it is difficult, if not impossible, to avoid peak insulin activity. Checking the pretreatment glucose with the patient’s glucometer, checking again during long procedures, and checking again at the end of the procedure provides a better understanding of the patient’s insulin pharmacodynamics and help prevent hypoglycemia.
TABLE 37-6
| ACTIVITY | ||||
| Type | Classification | Onset | Peak | Duration |
| Lispro; Aspart; Glulisine | Rapid acting | 15 minutes | 30-90 minutes | <5 hours |
| Regular | Short acting | 30-60 minutes | 2-3 hours | 4-12 hours |
| NPH | Intermediate acting | 2-4 hours | 4-10 hours | 14-18 hours |
| Levemir | Long acting | 1-2 hours | Relatively flat | up to 24 hours |
| Glargine | Long acting | 6-8 hours | “Peakless” (has no peak in activity) | 20-24+ hours |
If hypoglycemia occurs during dental treatment, therapy should be immediately terminated. If a glucometer is available, the blood glucose level should be checked. Treatment guidelines include the following41:
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


