33
Development and growth of the skull and age changes
Chapter contents
33.1 General principles of growth and development
The development of the facial bones is particularly important in the fields of paediatric dentistry and orthodontics. Dental students and dental practitioners who do not specialize in those subjects should have an appreciation of the subject to be aware of the changes to the face and jaws they are seeing in patients under continuous care as they grow, mature, and age.
Human beings increase in both size and complexity during the growth period which lasts from conception until maturity at about 16 to 18 years of age. As we have seen in Chapters 8, 13, 19, 21, and 32, most of the increase in complexity occurs during the pre- embryonic and embryonic phases of prenatal development although changes still occur in many organs and tissues well into post-natal life. Size increase is also rapid prenatally and continues throughout the remainder of the growth period although the growth rate changes. Changes in overall size may occur in mature individuals due to obesity or other pathological conditions but this is not growth.
33.1.2 Growth in overall proportions
Growth in overall size can be studied by examining the changes with age in easily measured parameters such as height and weight. There are two ways in which such data can be presented as shown in Figure 33.1. A distance curve is the simplest method illustrated in Figure 33.1A by plotting height against age on a graph. Changes in the rate of growth are demonstrated more clearly by plotting the increment in the measurement per unit of time such as the increase in height per year against age; this is a velocity curve shown in Figure 33.1B. You can see in Figure 33.1A that height increases more rapidly around the age of 14; the velocity curve in Figure 33.1B makes the rapid growth at this age much clearer.
If distance curves are plotted for different body components, the curves show specific characteristics. The overall growth of the body is accurately indicated by measures of height and weight; these measurements plotted against age produce the somatic growth curve shown in Figure 33.2. Growth is rapid in the prenatal and early post-natal period then begins to slow down after about 4 years of age. During the early teens starting about 11 years in girls and 13 years in boys, the gonads undergo maturation during puberty; this is followed almost immediately by the adolescent (or pubertal) growth spurt. During this growth spurt, height and weight increase rapidly for a few years and then slows again as maturity is approached. Bear in mind that growth may not be uniform and may proceed more rapidly in some directions than in others. The rate, timing, and direction determine the growth pattern.
Many other features of the body besides total height and weight follow the somatic growth pattern, including most of the internal viscera, the length of the limbs, and limb segments and their associated muscles and bones. Most significantly for dental students and practitioners, the facial skeleton also conforms to the somatic growth curve.
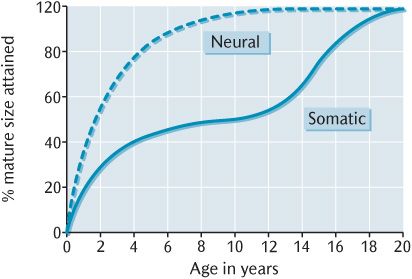
Particular organs or parts of the body have their own independent growth rate and time when growth occurs. An excellent and pertinent example is the neural growth curve followed by the brain and spinal cord, together with special sensory structures such as the eyeballs and organs of hearing, which is also shown in Figure 33.2. Observe how neural growth is rapid in prenatal and early post-natal life then declines smoothly and progressively; growth of the CNS and special sense organs is about 90% complete well before puberty around 8 years of age. In the context of skull growth, the cranial vault enclosing the brain, the upper facial skeleton forming the orbits around the eyeballs, and the petrous temporal bones enclosing the middle and inner ear follow the neural growth pattern.
The adolescent growth spurt
Growth hormone or somatotrophin secreted by the anterior pituitary gland is one of the principal factors governing the rate of somatic growth in the period before puberty. This hormone acts upon many target organs; in the current context, it is growth sites in the skeleton. Secretion of the sex hormones, testosterone and oestrogen, increases greatly as the gonads mature during puberty. These hormones are powerful stimulators of general body growth as well as their obvious influence on the development and growth of the primary and secondary sex organs. Their sudden increase at puberty produces the adolescent growth spurt. Sex hormones also bring about the maturation of growth sites and eventually the cessation of growth when they reach certain levels.
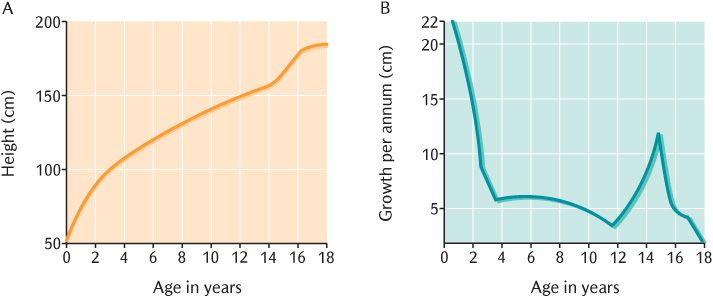
Fig. 33.1 A) Distance curve; B) Velocity curve for the growth of an individual.
Fig. 33.2 The somatic and neural growth curves.
There is little difference in body size or shape between males and females until puberty although females are usually a little bigger than males since they are physically more mature age for age. During adolescence, this trend is reversed because the adolescent growth spurt is more pronounced and prolonged in males than females because testosterones have a greater anabolic effect than oestrogens. The differential growth effects of the two sex hormones are also responsible for the development of sexual dimorphism in body shape which becomes increasingly marked during adolescence. The sex hormones also have differential effects upon many other features, including hair growth, disposition of body fat, muscular strength, and behavioural characteristics.
Maturation
Individuals of the same chronological age may differ from each other in their rate of growth, size attained, and degree of maturity. More mature children will have progressed further along their distance growth curve and therefore, tend to be taller than those who are less mature. In the early teens, one individual may be well into the adolescent growth spurt whereas a less mature individual of the same age may not yet have entered that phase. Assessment of maturity is outlined in Box 33.1.
Discrepancies between chronological age and maturity are important when assessing growth abnormalities. Retarded growth may be due to disorders of growth mechanisms or the result of delayed maturation. It is, therefore, sometimes necessary to assess maturity separately from growth using one of the indices of maturity. The centres of ossification in the eight wrist bones appear develop in a set sequence that can be visualized radiographically; their development is one the indices frequently used. The degree of development and eruption of the teeth is also a measure of maturity although it is not so reliable on its own as the ossification centres in the wrist. If ossification of the wrist bones is delayed, the eruption of teeth also tends to be retarded although not to the same degree.
33.2 Skull growth
33.2.1 Introduction
It is easy to think that the skeleton, including the skull, must be genetically determined to grow to a certain size. We have already seen how different tissues grow at different rates; these tissues can and do have a profound influence on growth and development of adjacent bones. Likewise, the influence of hormones on growth rates at different stages of maturity has been highlighted. It should now be coming apparent that several interrelated factors determine the growth of the skull and jaws. Some factors exert a general effect on the whole skeleton whereas others have a local influence on particular areas of the skeleton or even features of particular bones.
General effects which influence the overall shape and growth rate of bones include:
• Hormonal influences, particularly from growth and sex hormones;
• Nutritional influences; well-nourished people are usually taller and stronger than malnourished individuals;
• Genetic influences; children tend to have similar body build to one of their parents, but these characteristics are multifactorial with several genes involved;
• Socio-economic influences; children from lower socio-economic groups tend to be smaller than those from higher economic groups. Local effects include:
• The growth pattern followed by a particular part of the skull; the facial skeleton follows the somatic growth pattern, the cranial vault follows the neural growth pattern, and the cranial base follows the neural pattern for a certain time, then has its own patterns;
• The effect of enclosed tissues whereby the growth of adjacent bones follows the growth of the tissues they encapsulate. Such tissues are referred to as capsular matrices; the eyes and the brain are good examples as you will see in Section 33.4.
• Muscle attachments, joints, adjacent soft tissues, and teeth influence the detailed architecture of bones; these are sometimes referred to as periosteal matrices and their specific influence on the skull will be examined when their effects on particular bones are described.
These general and specific factors all determine the growth and development of the skull in various ways.
To briefly recapitulate some aspects of development and growth of the skull outlined in Section 22.2, the skull posterior to the coronal suture is derived from mesoderm whereas bones anterior to the suture are derived from ectomesenchyme from the neural crest. There are two types of bone formation and both start with coalescence of mesodermal or ectomesenchymal cells into dense groups, a process known as condensation. The condensed cells may differentiate into chondroblasts which form a cartilaginous template of the bone; this is endochondral ossification (ECO) and the cartilage is eventually replaced by bone. Some cartilages never ossify whereas other unmineralized residues of the initial cartilage template, such as the costal cartilages or xiphisternum, may partially ossify with age or pathologically. Intramembranous ossification (IMO) is bone formation directly in the condensation. In common with the whole of the post-cranial skeleton, the bones of the cranial base have cartilaginous precursors; the only exceptions are the clavicles. The bones of the cranial vault and facial skeleton are dermal bones which develop through IMO.
Most organs growth occurs by interstitial growth characterized by cell division and matrix proliferation throughout their structure. However, the mineralized matrix of bones precludes interstitial growth. Instead, bones grow by addition of new material on to pre-existing surfaces; this is appositional growth. Bones developing by ECO do not have the same limitations because it is the cartilage that is growing most actively during this process; cartilage can grow interstitially.
Irrespective of the mechanism of bone formation, the adult size and proportions of a bone are achieved by a combination of surface deposition and resorption, known collectively as remodelling. During the growth period, virtually all internal and external bone surfaces undergo deposition or resorption. Growth sites are surfaces or cartilages where particularly large amounts of growth take place.
33.2.2 Patterns of skull growth
There are numerous ways of measuring the pattern of skull growth. One method is to take measurements of skulls of different ages to produce cross-sectional growth data for a population. Measurements taken on the same child at successive intervals to follow the growth of that individual provide longitudinal data. Longitudinal data can be compared to cross-sectional data to determine whether the growth and development of an individual child is within normal limits for the population. Historically, skull growth was studied using lateral skull radiographs which had limitations due to distortion and superimposition of structures obscuring various features and landmarks. With the introduction of imaging methods described in Section 14.2, it has become much easier to obtain excellent three-dimensional images of growing skulls with virtually no distortion and no overlap of structures.
The development, growth, and age changes of the three major divisions of the skull will now be described in turn. The cranial base and facial skeleton are clinically the most important for dental students and practitioners, but the cranial vault illustrates some important principles of craniofacial development and growth. Briefly, the bones of the cranial vault are formed by IMO and follow the neural growth pattern; those of the facial skeleton undergo IMO and follow the somatic growth curve; the bones of the cranial base develop from cartilage through ECO and conform to the neural growth curve for several years, then adjust to the somatic growth pattern later.
The appearance of the head changes greatly with age. The brain-case has completed most of its growth before puberty whereas the facial skeleton does not complete growth until maturity because of the neural and somatic growth rates influencing the cranial vault and facial skeleton, respectively. The height of the cranial vault and orbit is about eight times that of the facial skeleton at birth due to the precocious prenatal growth of the brain and orbits. The cranial vault has reached about 60% of its adult size whereas the facial skeleton is on about 40% of its final size. This ratio reduces to about 6:1 in the second year and is about 5:1 by the fifth year; the face is comparatively small and facial features are undeveloped, giving the characteristic ‘childish’ appearance. There is an acceleration of growth in the facial skeleton at the onset of the adolescent growth spurt without any change in growth of the braincase; the head rapidly attains its adult proportions of 2.5:1 and the facial features become more strongly emphasized. The sex differences in facial appearance become fully established during adolescence; the male and female sex hormones have differential effects on the growth of the facial skeleton, the development of muscle and fat, and the growth of facial hair.
Facial appearance changes less rapidly in adult life. There is usually some increase in the heaviness of the features into middle age, resulting from continued small amounts of apposition growth in the facial skeleton and the accumulation of fat. In old age, the face tends to develop a shrunken appearance, partly from loss of bone from the facial skeleton, but more so from the wasting of the muscles of the face and age changes in skin, hair, and connective tissue. Loss of some or all of the teeth can also alter the aesthetic of the face.
33.3 The cranial base
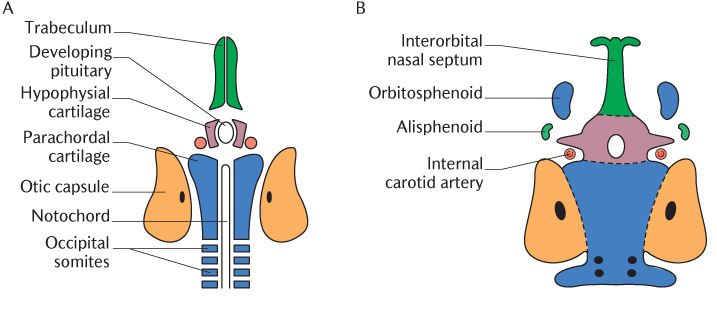
When we built a skull in Chapter 22, we started with the cranial base. This is how the skull is initiated during embryogenesis; the cartilaginous precursors of the cranial base appear before other elements of the skull. Cartilages begin to appear at about 7 weeks of development in the mesoderm or ectomesenchyme separating the brain above from the foregut below. Several small cartilages form a central stem and other cartilages outline paired lateral structures as shown in Figure 33.3A. The majority of dental courses now only require an outline of the process of development of the cranial base without naming of all the individual components; the names have been added to Figure 33.3 for those who require this information and for completeness.
In Figure 33.3A, identify the anterior end of the notochord in the posterior midline and the infundibulum, a pouch pushing down from the floor of the brain to form part of the pituitary gland. As shown in Figure 33.3A, two plates, one each side of the notochord, are the first element of the central stem to appear. They soon fuse across the mid-line, enclosing the front end of the notochord. The cartilage spreads backwards around the neural tube to form the foramen magnum. You should recognize in Figure 33.3C the outline of the basal part of the occipital bone although it does not look absolutely identical to the mature bone just yet.
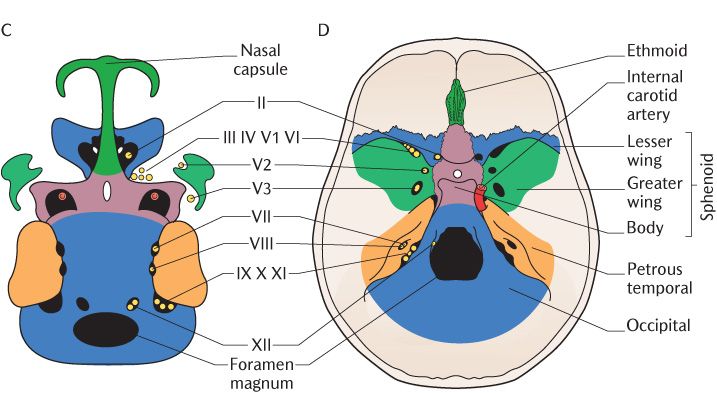
Fig. 33.3 A–C) The development of the main structural elements of the cranial base; D) Their contribution to the adult skull. Roman numerals refer to cranial nerves.
Figure 33.3A also shows a pair of smaller (hypophysial) cartilages on each side of the developing pituitary gland. These soon unite in front of and beneath the developing gland, creating the pituitary fossa in the body of the sphenoid bone; it meets the occipital bone posteriorly at the spheno-occipital synchondrosis indicated by the lower horizontal dotted line in Figure 33.3B. This synchondrosis is an important growth site.
During vertebrate evolution, three cartilaginous sense capsules originally developed lateral to the line of cartilages, forming the central stem of the cranial base to surround the nasal, optic, and otic sense organs. In mammals, the optic capsule does not develop into cartilage; it remains as connective tissue and forms the sclera of the eye. The otic capsule condenses around the developing middle and inner ear to form the petrous temporal bone. Figure 33.3C shows the growing otic capsule meeting the occipital bone posteriorly; the glossopharyngeal, vagus, and accessory nerves occupy the medial part of this junction and the jugular foramen is formed at that point.
Two (trabecular) cartilages extend anterior to the developing pituitary to form the future nasal septum illustrated in Figure 33.3A. Two nasal capsules develop around the nasal pits (Section 32.2.1) to form the nasal capsules and their medial walls fuse with the nasal septum in the midline; a large central aperture remains in the midline which later becomes the cribriform plate of the ethmoid bone for passage to the olfactory nerves.
The next stage of development is shown in Figure 33.3B. Two bilaterally paired cartilages develop in the region between the otic and nasal capsules (the orbitosphenoid anteriorly and the alisphenoid posteriorly). Each anterior cartilage becomes the lesser wing of the sphenoid, leaving the optic canal for the passage of the optic nerve as it unites with the body of the sphenoid. The posterior cartilage becomes the greater wing of the sphenoid. As this grows posteriorly, it encloses the maxillary and mandibular divisions of the trigeminal nerve in the foramen rotundum and foramen ovale, respectively. The superior orbital fissure is formed between the lesser and greater wings of the sphenoid; the oculomotor, trochlear and abducens nerves, and the ophthalmic division of the trigeminal nerve pass through the fissure into each orbit. The internal carotid artery is enclosed in the carotid canal where the greater wing joins with the body of the sphenoid and the tip of the petrous temporal bone. The cranial nerves and blood vessels supplying the brain pass to and from the cranial cavity through foramina between the central stem and the lateral structures; the foramina are formed between the developing components as they grow and fuse with each other as you can see in Figure 33.3C.
The chondrocranium is fully differentiated by the end of the second month of pregnancy, only 2 weeks since the initiation of cartilage formation. Mineralization of the chondrocranium takes place at various centres of ossification that appear in the cartilages of the cranial base and sense capsules; some form before the cranial base is completely mapped out. Figure 33.3D shows the appearance of the cranial base in the mature skull on to which the braincase and facial skeleton will be added. As we already know from earlier chapters, the cranial base comprises the ethmoid, sphenoid, temporal, and occipital bones. Note that the greater wings of the sphenoid have contributions from dermal bones forming the braincase and the pterygoid processes are dermal bones formed as part of the facial skeleton; irrespective of their origins, the seven components fuse together to form one complete sphenoid bone. The squamous parts of the temporal and occipital bones are also dermal bones.
The nasal capsules grow enormously in the early fetal period; their length doubles between 10 and 14 weeks as fusion of the facial processes occurs and grows to six times its original length by birth. The more posterior bones grow less quickly. The rapid growth of the nasal capsules is responsible for the change in the shape of the head in the late fetal period (see Section 33.3.2).
33.3.1 Synchondroses
As described in the previous section and illustrated in Figure 33.3, the growing cartilages of the cranial fuse with each other in various locations to map out the complex bones of the cranial base. The cartilaginous joints between these bones formed by remnants of this cartilage are called synchondroses. A typical synchondrosis is shown diagrammatically in Figure 33.4. A central progenitor zone containing undifferentiated cells is flanked on either side by proliferative zones in which the chondrocytes differentiate and divide. As the cells divide, they form into columns and deposit cartilage matrix in between. The/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses