CHAPTER 21
Osteoperiosteal Tissue-Engineered Injectable Bone
Opportunities multiply as they are seized.
—Sun Tzu
The use of dental implants in oral rehabilitation is becoming a standard method of care in den-A- tistry. When alveolar bone volume is insufficient to allow implant placement, augmentation is needed. The ability to augment the alveolar ridge has gradually expanded the scope of implant dentistry. During the past 10 years, alveolar augmentation techniques have become established treatment modalities. Dahlin et al1 reported an experimental study in rabbits involving the formation of new bone around titanium implants after use of a barrier membrane technique. Various bone grafting materials have been used for augmentation, including autogenous grafts, freeze-dried bone grafts, hydroxyapatite, and xenografts.2,3 Although the results of these reports indicate that various augmentations are clinically successful, this conclusion is questionable because only autogenous bone has osteogenic potential.4 However, use of autogenous bone is limited by availability for harvesting.
To avoid the need for graft harvesting of autogenous bone as well as the use of alloplasts alone, the authors attempted to regenerate bone in significant osseous defects with minimal invasiveness by providing a clinical alternative to the aforementioned graft materials that is both autologous and inductive. The new technology, termed tissue-engineered injectable bone,5,6 is based on tissue engineering concepts7 and involves the morphogenesis of new tissue using constructs formed from isolated autologous cells with biocompatible scaffolds and growth factors. It was previously reported that tissue-engineered bone induces excellent bone regeneration and promotes bone formation in a grafted area treated with platelet-rich plasma (PRP), which contains various growth factors.6
Recently, a two-step procedure for dental implant treatments, which involves placement of the implant after primary healing and remodeling of the graft, has been recommended for patients with less than 5 mm of alveolar bone height in the posterior maxilla or alveolar ridge. On the other hand, a one-step procedure, which allows dental implants to be placed simultaneously with grafts for patients who have at least 5 mm of alveolar bone to stabilize the implants,8–10 offers the advantages of fewer surgical treatments and the coordinated consolidation of the graft around the implants during healing, thus reducing both the number of surgical procedures and the healing time.
Therefore, the application of tissue-engineered bone plasticity for one-step procedures was explored by using mesenchymal stem cells (MSCs), PRP, and fibrin to increase the rate of bone formation and to enhance bone regeneration.
 Methods and Materials
Methods and Materials
Injectable bone preparation
The PRP was stored at 22°C in a conventional shaker until used. Human thrombin in a powder form (5,000 units) was dissolved in 5.0 mL of 10% calcium chloride in a separate sterile cup. Next, 3.5 mL of PRP, MSCs (1.0 × 107 cell/mL), and 0.5 mL of air were aspirated into a 5.0-mL sterile syringe. In a second 2.5-mL syringe, 500 µL of the thrombin–calcium chloride mixture was aspirated. The cells were resuspended directly into the PRP. The two syringes were connected with a T connector, and the plungers of the syringes were pushed and pulled alternately, allowing air bubbles to traverse the two syringes. Within 5 to 30 seconds, the contents assumed a gel-like consistency as the thrombin affected the polymerization of fibrin to produce an insoluble gel.
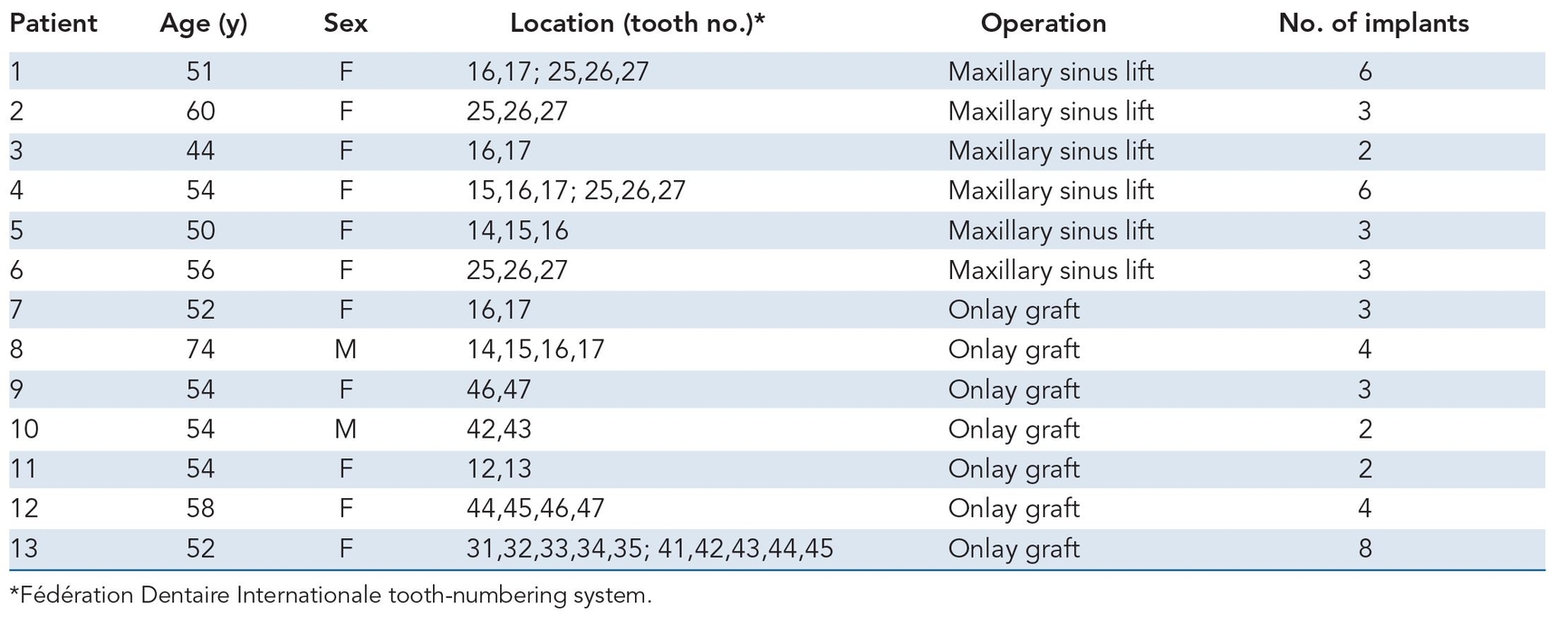
Table 21-1 Patient data
Patient selection
Thirteen patients aged 44 to 74 years (mean age of 54.6 years) were treated, and their responses were analyzed to determine the efficiency of tissue-engineered bone formation for alveolar ridge augmentation.
Six patients with partial or total edentulism received sinus floor grafting, and seven patients underwent concurrent onlay plasty in which vertical alveolar augmentation was performed. All patients had conventional denture retention problems because of severe anterior or posterior alveolar ridge atrophy. In the maxilla, some patients had a residual sinus floor of less than 5 mm in height so that sinus grafting and implants could resolve the problem (Table 21-1)12; in other patients, the residual alveolar arch was markedly atrophied in both a horizontal and sagittal dimension.
Patients were selected for injectable bone grafting because they preferred not to undergo any surgery for harvesting of autogenous bone. In all cases, the reconstruction included sinus floor grafting or onlay plasty with simultaneous implant replacement. All patients were healthy and free of disease that might influence treatment outcome (eg, diabetes, immunosuppressive chemotherapy, chronic sinus inflammation, or rheumatoid arthritis).
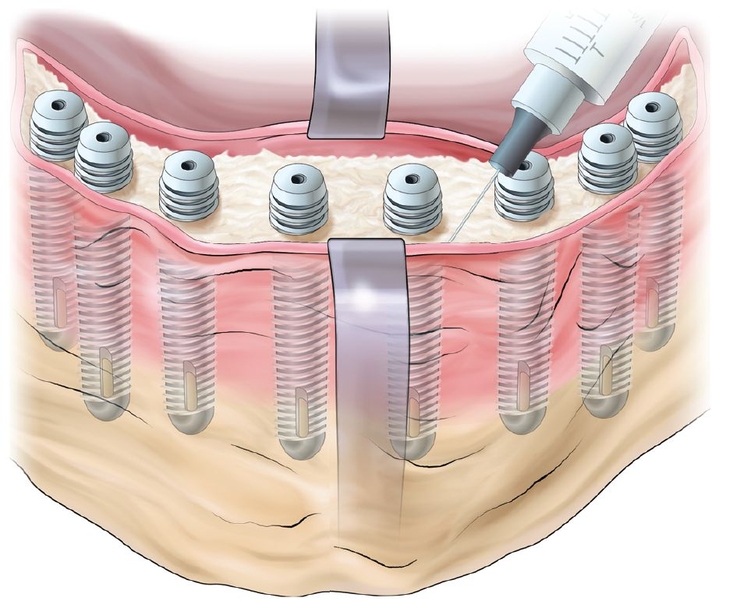
The patients were extensively informed about the risks and benefits of the prescribed procedures, including surgery, graft materials, implants, and uncertainties of using a new bone regenerative method (Fig 21-1). They consented to cooperate during treatment, and the research protocol was approved by the university ethics committee.
Surgical technique
Sinus floor augmentation
In all six patients, surgery was carried out while the patient was under general anesthesia. The sinus grafting procedure followed Tatum’s classic description.13 In brief, after elevation of a mucoperiosteal flap, a round hollow bur was used to create a door in the lateral maxillary sinus wall. After mobilization, the door was reflected inward. The space created by this procedure was filled with 1.5 to 5.8 g of tissue-engineered injectable bone. Simultaneous implant placement was performed. Care was taken to keep the sinus membrane intact to avoid spilling of the graft material. The mucoperiosteal flap was repositioned and sutured in the usual manner.
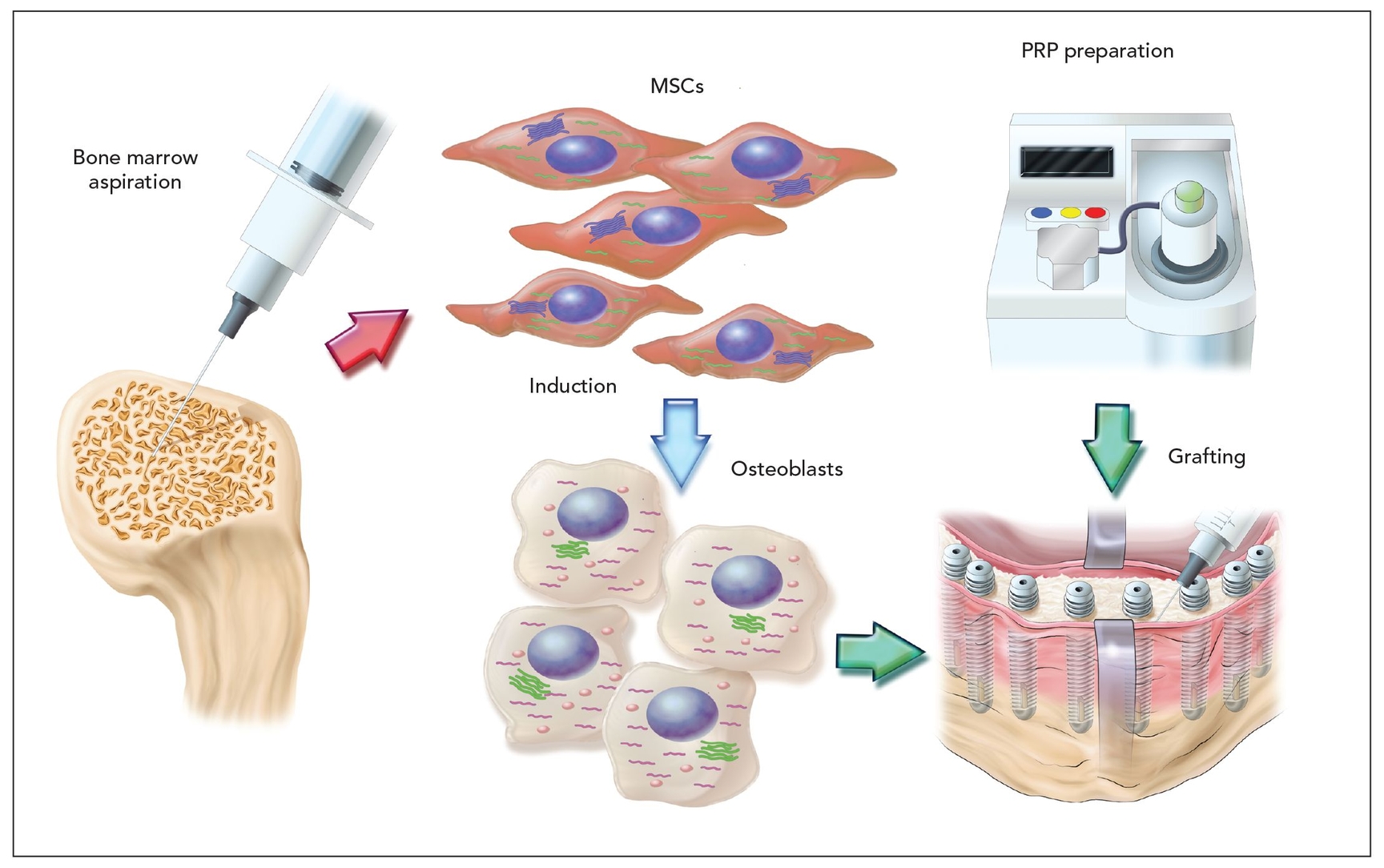
Fig 21-1 Protocol for preparation and injection of tissue-engineered bone. MSCs—mesenchymal stem cells; PRP—platelet-rich plasma.
 Results
Results
The MSCs were trypsinized at day 7 and used for the implants at a concentration of 1.0 × 107 cells/mL. The mean platelet count of the PRP was 972,269 (range of 524,480 to 2,033,000). These values confirmed the platelet sequestration ability of the process, which showed that the mean concentration was 446% above baseline.
None of the patients had postoperative problems besides normal swelling and inflammation at the surgical sites. The main complications during surgery were sinus membrane perforation and wound separation. Perforation of the sinus mucosa occurred in four procedures and resulted in only minor postoperative nasal bleeding without severe inflammatory signs during the observation period.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


