CHAPTER 19
Recombinant Protein Application for Bony and Periodontal Augmentation
Nullins in verba. (Not by word of mouth.)
—Royal Society of Science
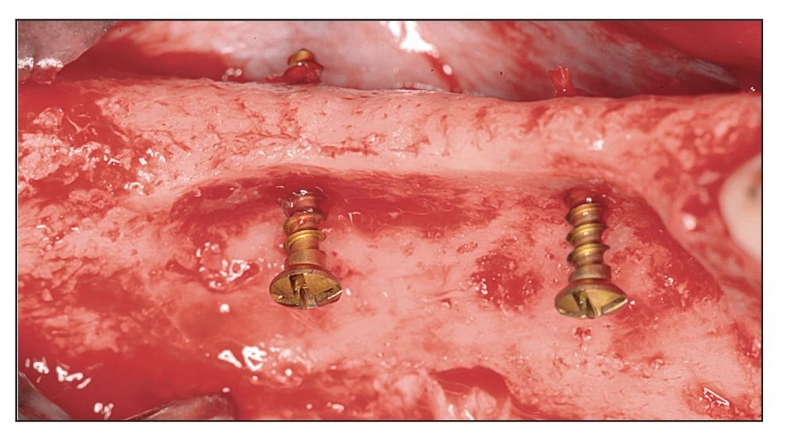
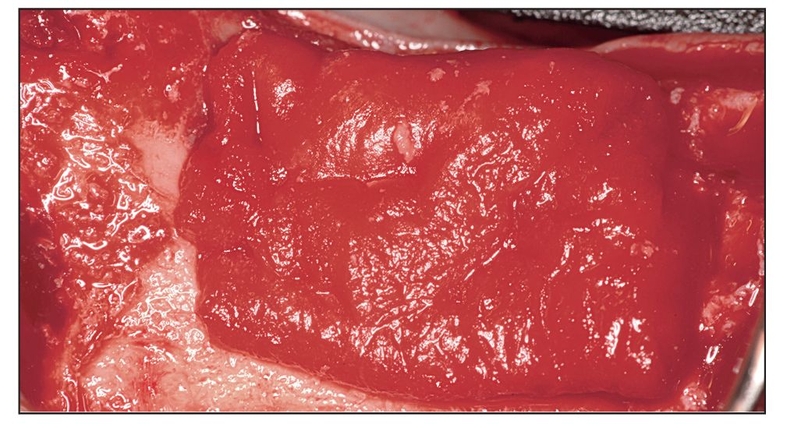
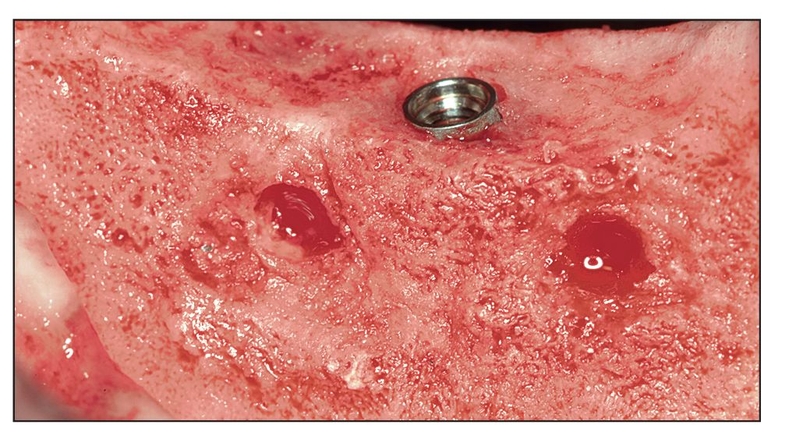
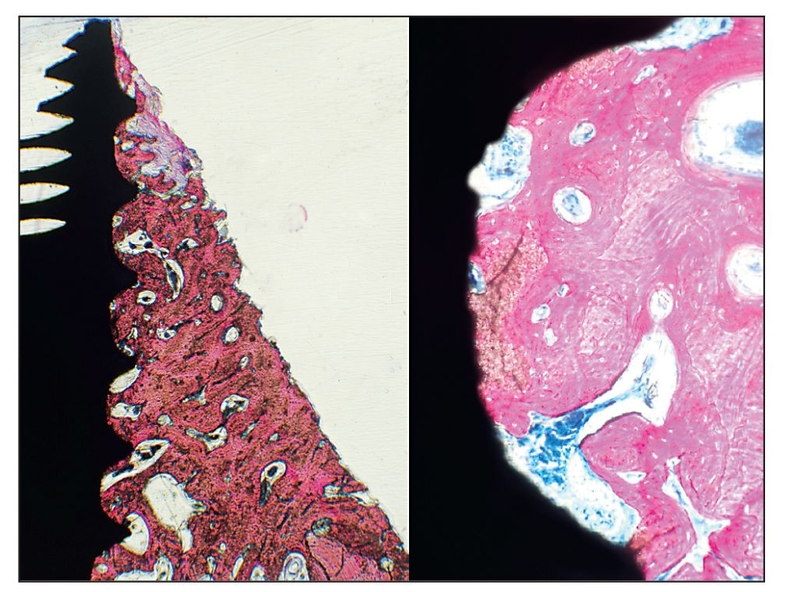
Alternatives to autogenous bone grafting include allografts, xenografts, and alloplasts, but only recently have recombinant protein adjuncts been available. Although natural bone graft materials contain inductive proteins, they either may not be present in sufficient quantity or may be incorporated in bone mineral and therefore inaccessible. If bone morphogenetic proteins (BMPs) inside the graft material are not readily released, they have no inductive effect. For example, proteins located inside organic bovine bone are inaccessible unless dissolved to isolate the BMP; otherwise, the action of the protein is not evident.1 The same is true for freeze-dried bone allograft and demineralized freeze-dried bone allograft. Therefore, subcutaneous placement of any of these materials in a human subject does not evoke a bone-forming response because inductivity is negligible.
To address this inadequacy with xenograft, a small protein peptide was added. The protein fragment had the ability to attach to cell surfaces, as demonstrated by column chromatography. Conceptually, the idea was to enhance cell binding to bone graft particles at the bone wound site, thereby stimulating bone growth. However, when this material was tested with actual cells in tissue culture conditions, enhanced cell attachment was negligible. There was almost no effect on cell binding after 15 minutes.2
More recently, complete proteins have been cloned that can be added to a host bone defect to stimulate bone growth. The protein must first be bound to a carrier so that the protein can be delivered to the wound site. A recombinant protein without a carrier is either not inductive or not retained long enough at the site to register a positive effect.
These proteins are made through recombinant technology and then combined with extrachromosomal DNA (plasmid), which allows that DNA to become incorporated into mammalian cell chromosomal DNA. In addition, another DNA sequence that can turn on and off the production of protein is usually added. In this way, the modified cells produce the protein, and the protein can be isolated from tissue culture medium. This process allows the production of large quantities of product such as recombinant human BMP (rhBMP). The molecular techniques use mammalian cells grown in tissue culture to make the protein. Similar techniques can also be employed using bacteria, which make the production of recombinant protein less expensive.
Several issues are extremely important if proteins are to be used to stimulate a healing process. First, the proteins must be adequately delivered to the wound site and must remain long enough to function without being degraded. This entails maintenance of a specific configuration in a highly specific tertiary and quaternary structure. Second, the proteins must adequately bind to their target cells. They must present in the correct configuration to their target cells, which are attracted to the wound site through chemotaxis. Third, the proteins must bind to the cells and effect a functional change in the activities of the cells. This usually requires that a specific membrane receptor be present on the cell wall and that, once the proteins bind to their respective receptors, a series of intracellular events occurs that results in a metabolic change.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses