Chapter 15
Adrenal Insufficiency
Background
The adrenal glands are small (6 to 8 g) endocrine glands located bilaterally at the superior pole of each kidney. Each gland contains an outer cortex and an inner medulla. The adrenal medulla functions as a sympathetic ganglion and secretes catecholamines, primarily epinephrine, whereas the adrenal cortex secretes several steroid hormones with multiple actions (< ?xml:namespace prefix = "mbp" />
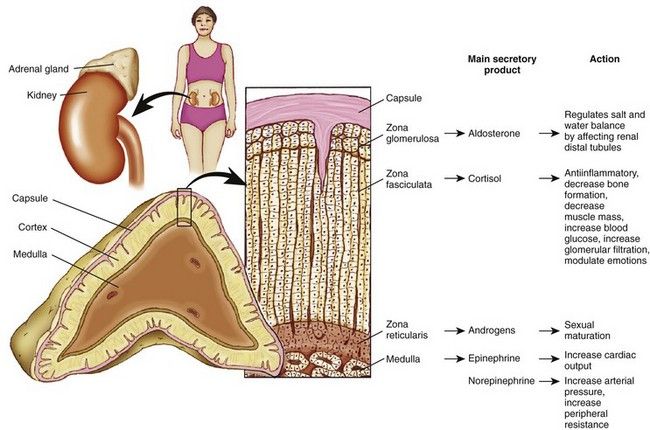
FIGURE 15-1 Structure of the adrenal gland, representative zones, and their main secretory products and physiologic actions.
(Adapted from Thibodeau GA, Patton KT: Anatomy and physiology, ed 7, St. Louis, 2010, Mosby.)
The adrenal cortex makes up about 90% of the gland and consists of three zones. The outer zone is the zona glomerulosa. The middle zone is the zona fasciculata, and the innermost zone is the zona reticularis. The cortex manufactures three classes of adrenal steroids: glucocorticoids, mineralocorticoids, and androgens. All are derived from cholesterol and share a common molecular nucleus. The predominant hormone of the zona glomerulosa is aldosterone, a mineralocorticoid. Aldosterone regulates physiologic levels of sodium and potassium and is relatively independent of pituitary gland feedback. The zona fasciculata secretes glucocorticoids, and the zona reticularis secretes androgens, or sex hormones.
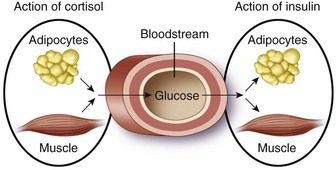
Cortisol, the primary glucocorticoid, has several important physiologic actions on metabolism, cardiovascular function, the immune system, and for maintaining homeostasis during periods of physical or emotional stress.
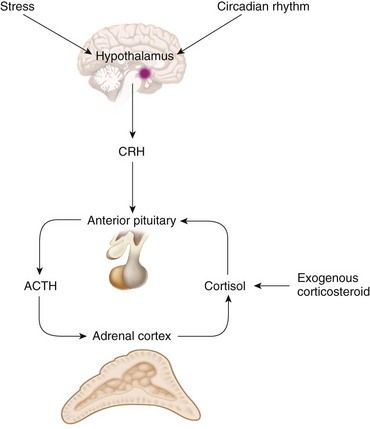
Regulation of cortisol secretion occurs through activity of the hypothalamic-pituitary-adrenal (HPA) axis (
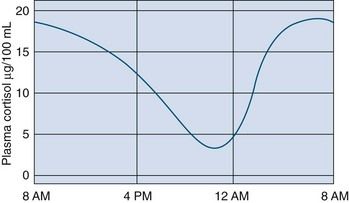
Cortisol secretion normally follows a diurnal pattern. Peak levels of plasma cortisol occur around the time of waking in the morning and are lowest in the evening and night
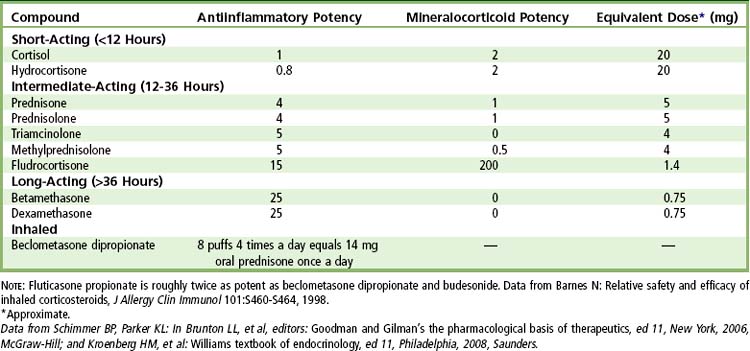
Synthetic glucocorticoids (cortisol-like drugs) are used in the treatment of many diseases (e.g., rheumatoid arthritis, systemic lupus erythematosus, asthma, hepatitis, inflammatory bowel disease, dermatoses, mucositis) and can affect adrenal function. Glucocorticoids are used on a long-term basis in patients during immunosuppressive therapy for organ transplantation and joint replacement. In dentistry, corticosteroids may be used during the perioperative period for the reduction of pain, edema, and trismus after oral surgical and endodontic procedures.
Mineralocorticoids
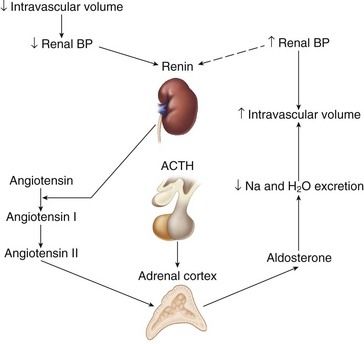
Aldosterone is the primary mineralocorticoid secreted by the adrenal cortex. It is essential to sodium and potassium balance and to the maintenance of extracellular fluid (i.e., intravascular volume). Its actions occur primarily on the distal tubule and the collecting duct of the kidney, where it promotes sodium and water retention, and potassium excretion. Aldosterone secretion is regulated by the renin-angiotensin system, ACTH, and plasma sodium and potassium levels. Aldosterone secretion is stimulated by a fall in renal blood pressure, which results from decreased intravascular volume or a sodium imbalance.
Adrenal Androgens
Dehydroepiandrosterone (DHEA) is the principal androgen secreted by the adrenal cortex. The effects of adrenal androgens are the same as those of testicular androgens (i.e., masculinization and the promotion of protein anabolism and growth). The activity of the adrenal androgens, however, is only about 20% that of the testicular androgens and is of relatively minor physiologic importance.
Definition
Disorders of the adrenal glands can result in overproduction (hyperadrenalism) or underproduction (hypoadrenalism or adrenal insufficiency) of adrenal products. Hyperadrenalism results from excessive secretion of adrenal cortisol, mineralocorticoids, androgens, or estrogen, in isolation or combination. The most common type of overproduction is due to glucocorticoid excess; when this is caused by pathophysiologic processes, the condition is known as Cushing’s disease.
Adrenal insufficiency is divided into two categories: primary and secondary. Primary adrenocortical insufficiency, also known as Addison’s disease, is characterized by destruction of the adrenal cortex with resulting deficiency of all of the adrenocortical hormones. The more common form, secondary adrenocortical insufficiency, may be the consequence of hypothalamic or pituitary disease, critical illness, or the administration of exogenous corticosteroids, with a deficiency of primarily cortisol. Both types of insufficiency downregulate adrenal production of cortisol. Inasmuch as abnormalities of adrenal function can render the patient medically compromised, these conditions are of significant concern in clinical practice.
Epidemiology
Incidence and Prevalence
Adrenal insufficiency occurs in 90 to 110 per 1 million persons of all ages, and diagnosis peaks in the fourth decade of life.
Etiology
Primary adrenocortical insufficiency is caused by progressive destruction of the adrenal cortex, usually because of autoimmune disease, chronic infectious disease (tuberculosis, human immunodeficiency virus [HIV] infection, cytomegalovirus infection, and fungal infection) or malignancy. The condition also may result from hemorrhage, sepsis, adrenalectomy, drugs, or genetic mutations (e.g., familial glucocorticoid deficiency).
Secondary adrenocortical insufficiency is a far more common problem and may be caused by structural lesions of the hypothalamus or pituitary gland (e.g., tumor), administration of exogenous corticosteroids, or less commonly, administration of specific drugs (e.g., desferrioxamine in the treatment of thalassemia) or a critical illness (burns, trauma, systemic infection).
Pathophysiology and Complications
The major hormones of the adrenal cortex are cortisol and aldosterone. Addison’s disease is caused by the lack of these compounds. Lack of cortisol results in impaired metabolism of glucose, fat, and protein, as well as hypotension, increased ACTH secretion, impaired fluid excretion, excessive pigmentation, and an inability to tolerate stress. The relationship between corticosteroids and response to stress involves the maintenance of vascular reactivity to vasoactive agents and the maintenance of normal blood pressure and cardiac output. Aldosterone deficiency results in an inability to conserve sodium and eliminate potassium and hydrogen ions, leading to hypovolemia, hyperkalemia, and acidosis.
Chronic excessive use of glucocorticoids can result in clinical features mimicking those of Cushing’s disease. This collection of clinical features of glucocorticoid excess is known as Cushing’s syndrome. This condition results from high levels of cortisol altering the proteins, carbohydrate and fat metabolism, the effects of insulin and vasculature homeostasis.
Corticosteroids that are topically applied or repeatedly locally injected or inhaled are rare inducers of adrenal suppression by absorption through the skin, subjacent tissues, or pulmonary alveoli.
Once corticosteroid administration has ceased, the HPA axis regains its responsiveness, and normal ACTH and cortisol secretion eventually resumes. The time required to regain normal adrenal responsiveness is thought to range from days to months. However, studies from a large review
Clinical Presentation
Signs and Symptoms
Hypoadrenalism
Signs and symptoms of the adrenal insufficiency are the result of deficiencies of adrenocortical hormones and often are nonspecific, leading to delays in diagnosis. Clinical evidence of deficiency generally appears only after 90% of the adrenal cortices have been destroyed.
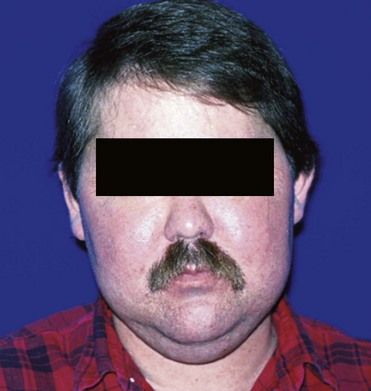
Primary adrenal insufficiency (Addison’s disease) produces signs and symptoms that relate to a deficiency of aldosterone and cortisol. The most common complaints are weakness, fatigue, abdominal pain, and hyperpigmentation of the skin and mucous membranes (
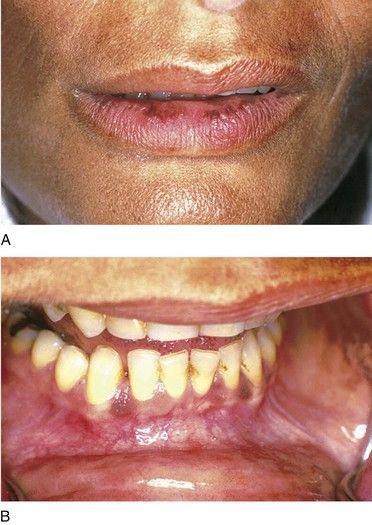
FIGURE 15-6 Patient with Addison’s disease. Note bronzing of the skin with pigmentation of the lip (A) and the oral mucosa (B).
Secondary adrenal insufficiency caused by long-term corticosteroid administration may cause a partial insufficiency that is limited to glucocorticoids. The condition usually does not produce any symptoms unless the patient is significantly stressed and does not have adequate circulating cortisol during times surrounding stress. In this event, an adrenal crisis is possible. However, an adrenal crisis in a patient with secondary adrenal suppression is rare and tends not to be as severe as that seen with primary adrenal insufficiency, because aldosterone secretion is normal. Thus, hypotension, dehydration, and shock are seldom encountered.
Hyperadrenalism
Adrenal hyperfunction can produce four syndromes that are dependent on the adrenal product that is in excess—androgen, estrogen, mineralocorticoid, and cortisol. Androgen-related disorders are rare and primarily affect the reproductive organs. Mineralocorticoid excess (primary aldosteronism) is associated with hypertension, hypokalemia, and dependent edema (see
Laboratory Findings
Laboratory assessment for adrenal insufficiency generally is initiated with a provocative stimulation test of the HPA axis. These tests include the synthetic ACTH (cosyntropin) stimulation test, the CRH test, and the dexamethasone suppression test. The ACTH stimulation test is the most reliable and most commonly used test of the three. It is carried out by injecting a synthetic ACTH hormone, such/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses