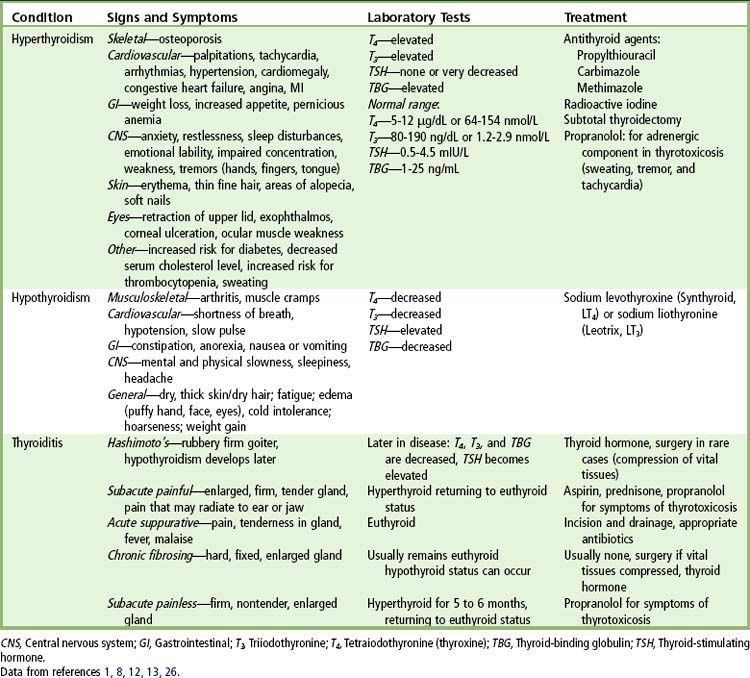
Chapter 16
Thyroid Diseases
Thyroid disease in a patient who presents for dental treatment is a cause for concern on several fronts. Undiagnosed or poorly controlled disorders of the thyroid can be expected to compromise outcomes with otherwise perfectly appropriate dental management plans. Detection of early signs and symptoms of such disorders during the dentist’s initial assessment, however, can lead to referral of the patient for medical evaluation and treatment. In some instances, such intervention may be lifesaving, whereas in others, quality of life can be improved and complications of certain thyroid disorders avoided, particularly in the context of delivery of dental care.
The focus of this chapter is on disorders involving hyperfunction of the gland (hyperthyroidism or thyrotoxicosis), hypofunction of the gland (hypothyroidism or myxedema or cretinism), thyroiditis, and the detection of lesions that may be cancerous (< ?xml:namespace prefix = "mbp" />
TABLE 16-1 Etiology of Thyroid Conditions
| Thyroid Condition | Causes |
|---|---|
| Hyperthyroidism | |
| Hypothyroidism (cretinism, myxedema) |
|
| Thyroiditis | |
| Thyroid neoplasms |
hCG, Human chorionic gonadotropin; TSH, Thyroid-stimulating hormone.
Thyroid Gland
Location
The thyroid gland, which is located in the anterior portion of the neck just below and bilateral to the thyroid cartilage, develops from the thyroglossal duct and portions of the ultimobranchial body
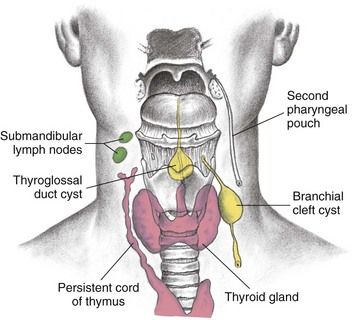
FIGURE 16-1 Thyroglossal duct cyst and branchial cleft cyst development.
(From Seidel HM, et al: Mosby’s guide to physical examination, ed 7, St. Louis, 2011, Mosby.)
The parathyroid glands develop from the third and fourth pharyngeal pouches and become embedded within the thyroid gland.
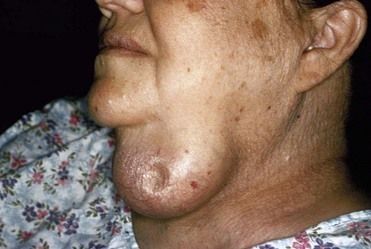
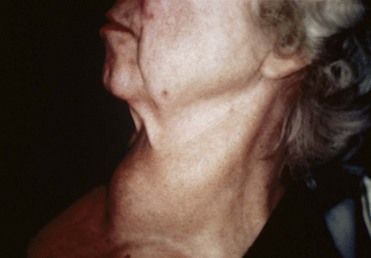
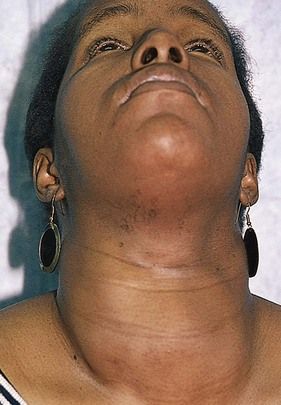
Enlargement and Nodules of the Thyroid Gland
Generalized enlargement of the thyroid gland, referred to as a goiter, may be diffuse (
Function of the Thyroid Gland
The thyroid gland secretes three hormones: thyroxine (T4), triiodothyronine (T3), and calcitonin.
Most thyroid actions (metabolic and developmental) are mediated through activity of nuclear receptors that are tissue site–specific.
Calcitonin is involved, along with parathyroid hormone and vitamin D, in regulating serum calcium and phosphorus levels and skeletal remodeling. (This hormone and its actions are considered further in
Epidemiology
Incidence and Prevalence
Graves’ disease occurs in up to 2% of women and 0.2% of men. Graves’ disease is rare before adolescence; the usual age at presentation is between 20 and 50 years, although it does occur in elderly persons.
Subacute painful thyroiditis accounts for 5% of all medical consultations regarding thyroid disorders, and is three times more common in women than men. Subacute painless thyroiditis occurs in patients with underlying autoimmune thyroid disease and is reported in up to 5% of women 3 to 6 months after pregnancy. In these circumstances it is called postpartum thyroiditis. Riedel’s thyroiditis is a rare form of chronic thyroiditis that typically occurs in middle-aged women. Acute suppurative thyroiditis is rare.
Thyroid nodules can be found in about 5% of the adult population in the United States.
Pathophysiology and Etiology
Blood levels of T4 and T3 are controlled through a servofeedback mechanism mediated by the hypothalamic-pituitary-thyroid axis (
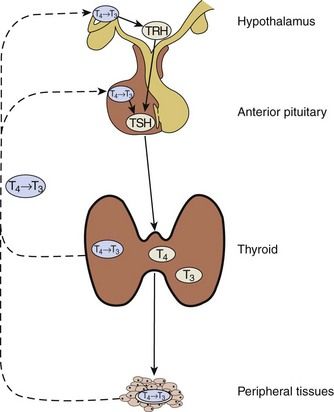
FIGURE 16-6 Hypothalamic-Pituitary-Thyroid Axis.
Solid lines correspond to stimulatory effects, and dotted lines depict inhibitory effects. Conversion of T4 to T3 in the pituitary and the hypothalamus is mediated by 5′-deiodinase type II. This event also is important throughout the central nervous system, thyroid, and muscle. 5′-Deiodinase type I (propylthiouracil-sensitive) plays a major role in liver, kidney, and thyroid function. TRH, Thyrotropin-releasing hormone; TSH, Thyroid-stimulating hormone.
(Redrawn from DeGroot LJ, Jameson JL: Endocrinology, ed 5, vol 2, Philadelphia, 2006, Saunders.)
Under normal conditions, thyrotropin-releasing hormone (TRH) is released by the hypothalamus in response to external stimuli (e.g., stress, illness, metabolic demand, low levels of T3 and, to a lesser extent, T4). TRH stimulates the pituitary to release thyroid-stimulating hormone (TSH), which causes the thyroid gland to secrete T4 and T3. T4 and T3 also have a direct influence on the pituitary. High levels turn off the release of TSH, and low levels turn it on. In the blood, T4 and T3 are almost entirely bound to plasma proteins.
Binding plasma proteins consist of thyroxine-binding globulin (TBG), transthyretin, and thyroid-binding albumin (TBA). Small amounts of T3 and T4 are bound to high-density lipoproteins.
Low T4 and T3 plasma levels often are found in ill and medicated older persons. Protein abnormalities can affect total T4 and T3 levels. Illness can reduce the conversion of T4 to T3. Drugs and illness also can affect free levels of T4 and T3. The main age-related change seen in much older individuals is a fall in T3 due to the reduced peripheral conversion of T4 to T3.
Antibodies to various structures within the thyroid are associated with autoimmune diseases of the thyroid. Graves’ disease and Hashimoto’s thyroiditis have such an association. Three autoantibodies are most often involved in autoimmune thyroid disease: TSH receptor antibodies (TSHRAb), thyroid peroxidase antibodies (TPoAb), and thyroglobulin antibodies (TgAb).
TgAb are found in about 10% to 20% of the general population. These antibodies are present in 50% to 70% of patients with Graves’ disease and in 80% to 90% of those with autoimmune thyroiditis.
Laboratory Tests
Direct tests of thyroid function involve the administration of radioactive iodine. Measurement of thyroid radioactive iodine uptake (RAIU) is the most common of these tests. 131I has been used for this test, but 123I is preferred because it exposes the patient to a lower radiation dose. RAIU, which is measured 24 hours after administration of the isotope, varies inversely with plasma iodide concentration and directly with the functional status of the thyroid. In the United States, normal 24-hour RAIU is 10% to 30%. RAIU discriminates poorly between normal and hypothyroid states. Values above the normal range usually indicate thyroid hyperfunction.
Several tests are available that measure thyroid hormone concentration and binding in blood. Highly specific and sensitive radioimmunoassays are used most often to measure serum T4 and T3 concentrations and rarely to measure reverse T3 (rT3) concentration. The normal range for T4 is 64 to 154 nmol/L (5 to 12 µg/dL). The normal range for T3 is 1.2 to 2.9 nmol/L (80 to 190 ng/dL).
Measurement of basal serum TSH concentration is useful in the diagnosis of hyperthyroidism and hypothyroidism. Very sensitive methods, such as immunoradiometric or chemiluminescent techniques, are now available to measure serum TSH. The normal range for TSH is 0.5 to 4.5 mIU/L (µIU/mL). In cases of hyperthyroidism, the TSH level is almost always low or nondetectable. Higher levels indicate hypothyroidism
| Test | Normal Range | Interpretation |
|---|---|---|
| Radioactive iodine uptake (RIU) | 5-30% | Elevated: hyperthyroidism Decreased: hypothyroidism |
| Thyroid-stimulating hormone (TSH) | 0.5-4.5 mIU/L | Elevated: hypothyroidism Suppressed: hyperthyroidism |
| Total serum T4 (TT4) | 5-12 µg/dL 64-154 nmol/L |
High: hyperthyroidism Low: hypothyroidism |
| Free T4 (FT4) | 1.0-3.0 ng/dL 13-39 pmol/L |
Increased: hyperthyroidism Decreased: hypothyroidism |
| Total serum T3 (TT3) | 1.2-2.9 nmol/L 80-190 ng/dL |
High: hyperthyroidism Low: hypothyroidism |
| Free T3 (FT3) | 0.25-0.65 ng/dL 3.8-10 nmol/L |
Increased: hyperthyroidism Decreased: hypothyroidism |
Other tests used in selected cases include the TSH stimulation test, the T3 suppression test, and radioassay techniques for measuring TSHRAb, TSHR-blocking Ab, TPoAb, and TgAb.
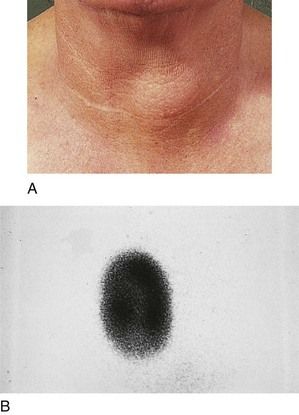
Ultrasonography may be used to detect thyroid lesions. Nodules 1 to 2 mm in size can be identified. This technique also is used to distinguish solid from cystic lesions, to measure the gland, and to guide needles for aspiration of cysts or for biopsy of thyroid masses. Computed tomography (CT) and magnetic resonance imaging (MRI) are helpful mainly in the postoperative management of patients with thyroid cancer. These forms of imaging also are used for the preoperative evaluation of larger lesions of the thyroid (greater than 3 cm in diameter) that extend beyond the gland into adjacent tissues.
Thyrotoxicosis (Hyperthyroidism)
Etiology, Pathophysiology, and Complications
The term thyrotoxicosis refers to an excess of T4 and T3 in the bloodstream. This excess may be the result of production by ectopic thyroid tissue, multinodular goiter, or thyroid adenoma or may be associated with subacute thyroiditis (painful and painless), ingestion of thyroid hormone (thyrotoxicosis factitia) or of foodstuffs containing thyroid hormone, or pituitary disease involving the anterior portion of the gland (see
Graves’ disease is an autoimmune disease in which thyroid-stimulating immunoglobulins bind to and activate thyrotrophic receptors, causing the gland to grow and stimulating the thyroid follicles to increase the synthesis of thyroid hormone.
Clinical Presentation
Signs and Symptoms
Direct and indirect effects of excessive thyroid hormones contribute to the clinical picture in Graves’ disease. The most common symptoms and signs are nervousness, fatigue, rapid heartbeat or palpitations, heat intolerance, and weight loss (
Graves’ ophthalmopathy, which is identified in approximately 50% of patients, is characterized by edema and inflammation of the extraocular muscles, as well as an increase in orbital connective tissue and fat. Ophthalmopathy is an organ-specific autoimmune process that is strongly linked to Graves’ hyperthyroidism. Although hyperthyroidism may be successfully treated, ophthalmopathy often produces the greatest long-term disability for patients with this disease.
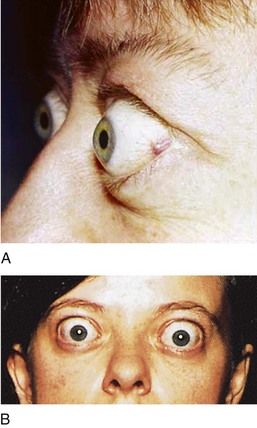
FIGURE 16-7 Eyelid changes in Graves’ disease. A, Lid retraction is a common eye sign in Graves’ disease. It is recognized when the sclera is visible between the lower margin of the upper lid and the cornea. B, Proptosis in Graves’ disease results from enlargement of muscles and fat within the orbit as a result of mucopolysaccharide infiltration.
(A, From Goldman L, Ausiello D: Cecil textbook of medicine, ed 23, Philadelphia, 2008, Saunders. B, From Seidel H: Mosby’s guide to physical examination, 4th ed 4, St. Louis, 1999, Mosby.)
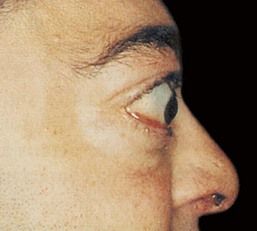
FIGURE 16-8 Exophthalmos of Graves’ disease can be unilateral or bilateral. The forward protrusion of the globe results from an increase in volume of the orbital contents.
(From Stein HA, Stein RM, Freeman MI: The ophthalmic assistant: a text for allied and associated ophthalmic personnel, ed 8, Philadelphia, 2006, Mosby.)
Most thyrotoxic patients show eye signs not related to the ophthalmopathy of Graves’ disease as well. These signs (i.e., stare with widened palpebral fissures, infrequent blinking, lid lag, jerky movements of the lids, and failure to wrinkle the brow on upward gaze) result from sympathetic overstimulation and usually clear when thyrotoxicosis is corrected.
Another complication, which is found in about 1% to 2% of patients with Graves’ disease, is dermopathy (
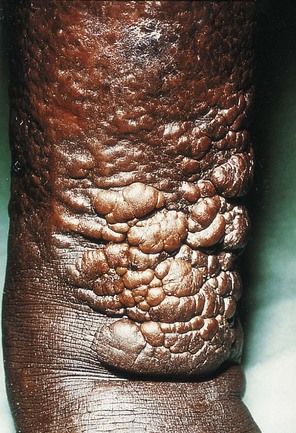
FIGURE 16-9 Infiltrative dermopathy seen in Graves’ disease. Hyperpigmented, nonpitting induration of the skin of the legs usually is found in the pretibial area (pretibial myxedema). Lesions are firm, and clear edges can be seen.
(From Melmed S, et al: Williams textbook of endocrinology, ed 12, Philadelphia, 2011, Saunders. Courtesy Dr. Andrew Werner, New York, New York.)
Thyroid acropachy is another rare manifestation of Graves’ disease. This feature is associated with presence of TgAbs (
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses