This type of cleavage is favored by light and heat and forms free radicals.
Heterolytic cleavage, which is a deprotonation reaction leaving an electron pair:
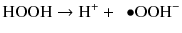
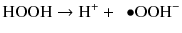
This deprotonation occurs at increased pH and generates perhydroxyl anions (Feinman et al. 1991).
A third pathway is derived by a combination of homolytic and heterolytic cleavage and generates active oxygen that is both an anion and a free radical:
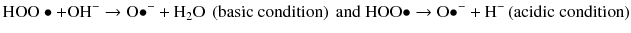
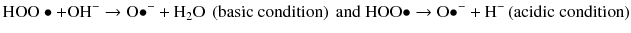
Active oxygen is attracted to electron-rich areas of stain molecules and cleaves double bond to reduce color or remove the compound (Albers 1991).
Despite the well-known chemistry of hydrogen peroxide and its application to establish the chromophore theory, many issues remain unsolved. Studies using Fourier Transform Infra-Red (FTIR) and Raman spectroscopies failed to detect chromophores or their breakdown products in the enamel, and are inconsistent with the chromophore theory (Darchuk et al. 2008; Eimar et al. 2012; Fattibene et al. 2005). Thus, continued investigation is required to fully understand the mechanisms of hydrogen peroxide in eliminating stains.
Ideally, as hydrogen peroxide moves from the external tooth surface into the enamel and dentin, its oxidizing action should be limited to organic chromophores until it reaches a certain saturation point, or whitening threshold. Oxidizing action beyond the whitening threshold—characterized by the depletion of chromophores—has been cautioned against as it might compromise tooth structure. Indeed, review of the literature suggests hydrogen peroxide has significant interactions with the organic and inorganic components of enamel and dentin well before the saturation point. This may account for alterations in the physical properties of the tooth substrate after the whitening treatment (Attin et al. 2005).
Extensive studies using ion-selective electrode probes, FT-Raman spectroscopy, and a combination of scanning electron microscopy (SEM) and energy-dispersive X-ray spectrometer and microcomputerized tomography suggest that hydrogen peroxide interacts with the tooth structure, and changes the chemical composition of enamel and dentin (Kwon and Wertz 2016). While evidence exists that peroxide-based materials do not irreversibly influence the chemistry of enamel and dentin beyond clinical relevance (Arcari et al. 2005; Cavalli et al. 2011; Goo et al. 2004; Lee et al. 2006; Mc Cracken and Haywood 1996; Rodrigues et al. 2007), several studies have demonstrated significant changes in the calcium/phosphate ratio, indicative of alterations in the inorganic components of hydroxyapatite (Al-Saleni et al. 2007; Berger et al. 2010; Bizhang et al. 2006; de Freitas et al. 2004; Efeoglu et al. 2005; Efeoglu et al. 2007; Rotstein et al. 1996; Rotstein et al. 1992). Microcomputerized tomography studies of enamel treated with 10 % or 35 % carbamide peroxide demonstrated a demineralization depth of 50 μm and 250 μm, respectively (Efeoglu et al. 2005; Efeoglu et al. 2007). Furthermore, infrared spectroscopic analysis showed changes in the enamel that was both concentration and time dependent.
It is worth noting that changes in the organic component of enamel and dentin are likely due to the oxidizing ability of hydrogen peroxide, while changes in the mineral component are mainly attributed to its acidity (Jiang et al. 2007). Several studies provide evidence supporting that the organic matrix of enamel and dentin is oxidized by hydrogen peroxide. X-ray diffraction analysis of hydroxyapatite suggests hydrogen peroxide influences the organic tissue, and Nuclear Magnetic Resonance-based measurements indicate that proline and alanine may be more susceptible to an attack by the hydroxyl radical (Kawamoto and Tsujimoto 2004; Sato et al. 2013; Toledano et al. 2011). Other studies assessing morphological changes in the enamel and dentin used atomic force microscopy (AFM) and FTIR to show that the tooth enamel matrix protein or organic matrix of dentin had partially lysed, causing these effects (Abouassi et al. 2011; Chng et al. 2005; Hegedüs et al. 1999; Mahringer et al. 2009; Sato et al. 2013; Ubaldini et al. 2013). Moreover, other studies have implicated that proteolysis by dentin metalloproteinases and cathepsin B might also compromise the organic component of dentin (Sato et al. 2013; Toledano et al. 2011).
Collectively, these studies demonstrate that hydrogen peroxide indeed interacts with all components of dentin and enamel. Thus, it may not only target chromophore stains, but also whiten by modifying the organic substances within the tooth. Future studies must identify the clinical significance of interactions between hydrogen peroxide and each tooth layer.
2.2.3 Phase Three: Surface Change and Color
The anticipated final outcome of tooth whitening is to increase color lightness and reduce chroma in the yellow-blue and red-green spectrum based on the CIE Lab system (Commission Internationale de l’Eclairage 1995). The separate contributions of enamel and dentin on tooth color have been evaluated, with some studies placing more emphasis on the role of dentin (Kwon et al. 2013; Wiegand et al. 2005; Kugel et al. 2007). Nevertheless, enamel characteristics also play a key role in the optical properties of the tooth. Enamel contributes to the overall tooth color by decreasing the translucency of the tooth, masking the color of the underlying dentin (Kawamoto and Tsujimoto 2004; Ma et al. 2009, 2011). Changes in the enamel have been attributed to micromorphological alterations through deproteinization, demineralization, and oxidation of the most superficial enamel layer (Eimar et al. 2011; Ma et al. 2009, 2011). This changes the density of enamel making the distribution of enamel crystals less compact and potentially increasing its refractive index (Li et al. 2010; Ma et al. 2011).
Determining how subtle enamel surface changes affect the tooth has been an area of interest. Studies have found that rough surfaces create a more diffuse reflection, turning the object brighter, whereas a smooth surface leads to more specular reflection. Additionally, an increase in back scattering of short wavelengths, reflected as bluish-white, plays a considerable role in the light scattering of teeth (Joiner 2004). This is most easily demonstrated by the whitish color change in early caries lesions due to the increased opacity of the tooth enamel (Ma et al. 2009, 2011; Vieira et al. 2008). Further, some studies suggest tooth color change that is associated with tooth whitening is mainly due to mineral loss rather than the breakdown of chromophores (Jiang et al. 2007; Kwon et al. 2002; Lee et al. 2006; Mc Cracken and Haywood 1996). The subsequent uptake of minerals after tooth whitening and the reversal of the treatment substantially support this suggestion (Li et al. 2010).
Because of the impact of surface changes on the appearance of tooth color, changes in surface topography have been extensively investigated. SEM and AFM studies showed increased roughness and surface irregularities upon whitening treatment (Ben-Amar et al. 1995; Bitter and Sanders 1993; Hosoya et al. 2003; McGuckin et al. 1992; Pedreira De Freitas et al. 2010; Pinto et al. 2004; Shannon et al. 1993; Yeh et al. 2005; Zalkind et al. 1996). Notably, most of these changes have not been seen in studies where a remineralizing agent or saliva was used as a storage medium (Duschner et al. 2006; Haywood et al. 1991; Joiner et al. 2004; Scherer et al. 1991; Turkun et al. 2002; White et al. 2003). Thus, continued research on the effects of whitening treatments on surface and tooth color changes are necessary to order to prescribe treatments that will have long-lasting effects with minimal changes to the overall structure of the tooth.
This up-to-date review of the literature illustrates that tooth whitening occurs in three distinct phases, challenging the validity of the widely accepted “chromophore effect” as the dominant mechanism of hydrogen peroxide. As such, this theory must be modified to reflect the true complexity of the mechanisms that drive whitening. Indeed, stains are not determined by the properties of the organic staining molecules alone but are also affected by micromorphologic alterations on the tooth surface and within the tooth structure; thus, whitening likely affects intact enamel and dentin microstructures, an underrecognized concern (Kwon and Wertz 2016). In future studies, an appreciation of the complexity of the tooth whitening process will spearhead innovation toward materials and techniques that meet the ever-growing interest in safely obtaining a brighter smile.
References
Abouassi T, Wolkewitz M, Hahn P (2011) Effect of carbamide peroxide and hydrogen peroxide on enamel enamel surface: an in vitro study. Clin Oral Investig 15:673–680PubMed
Ake-Linden L (1968) Microscopic observations of fluid flow through enamel in vitro. Department of Oral Histopathology, Karolinska Institute, School of Dentistry, Stockholm, Sweden Report Nr Op.R(4).
Al-Saleni SK, Wood DJ, Hatton PV (2007) The effect of 24h non-stop hydrogen peroxide concentration on bovine enamel and dentin mineral content and microhardness. J Dent 35:845–850
Albers H (1991) Lightening natural teeth. ADEPT Report 2:1–24
Arcari GM, Baratieri LN, Maia HP, De Freitas SF (2005) Influence of the duration of treatment using a 10% carbamide peroxide bleaching gel on dentin surface microhardness: an in situ study. Quintessence Int 36:15–24PubMed
Attin T, Vollmer D, Wiegand A, Attin R, Betke H (2005) Subsurface microhardness of enamel and dentin after different external bleaching procedures. Am J Dent 18:8–12PubMed
Bartels HA (1939) A note on chromogenic microorganism from an organic colored deposit of the teeth. Int J Orthod 25:795
Ben-Amar A, Liberman R, Gorfil C, Bernstein Y (1995) Effect of mouthgurad bleaching on enamel surface. Am J Dent 8:29–32PubMed
Benetti AR, Valera MC, Mancini MN, Miranda CB, Balducci I (2004) In vitro penetration of bleaching agents into the pulp chamber. Int Endod J 37:120–124PubMed
Berger SB, Cavalli V, Martin AA, Soares LE, Arruda MA, Brancalion ML, Giannini M (2010) Effects of combined use of light irradiation and 35% hydrogen peroxide for dental bleaching on human enamel mineral content. Photomed Laser Surg 28:533–538PubMed
Bevelander G (1964) The effect of tetracycline on mineralization and growth. Adv Oral Biol 1:205–223PubMed
Bharti R, Wadhwani K (2013) Spectrophotometric evaluation of peroxide penetration into the pulp chamber from whitening strips and gel: an in vitro study. J Conserv Dent 16:131–134PubMedPubMedCentral
Bitter NC, Sanders JL (1993) The effect of four bleaching agents on the enamel surface: a scanning electron microscopic study. Quintessence Int 24:817–824PubMed
Bizhang M, Seemann R, Duve G, Römhild G, Altenburger JM, Jahn KR, Zimmer S (2006) Demineralization effects of 2 bleaching procedures on enamel surfaces with and without post-treatment fluoride application. Oper Dent 31:705–709PubMed
Bowles WH, Ugwuneri Z (1987) Pulp chamber penetration by hydrogen peroxide following vital bleaching procedures. J Endod 13:375–377PubMed
Brotherton Boron BJ (1994) Inorganic chemistry encyclopedia of inorganic chemistry. John Wiley & Sons, Bruce King
Budavari S, O’Neill MJ, Smith A, Heckelman PE (1989) The merck index: an encyclopedia of chemicals, drugs, and biologicals. Merck and Co., Inc, Rahway
Burt BA (1992) The changing patterns of systemic fluoride intake. J Dent Res 71:1228–1237PubMed
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


