(1)
Department of Pediatric Dentistry, Selcuk University, Faculty of Dentistry, Konya, Turkey
Abstract
Experimental research on tooth development or odontogenesis is based very largely on the teeth of murine rodents (Butler 1967). Pioneering work by Shirley Glasstone on rat tooth germ cultures gave detailed information about structural, histological, and self-differentiating interactions of explants (Glasstone 1936). However, the dentition of the mouse and that of many other mammals is very different. Mice have highly specialized incisors, distinctive molar patterns, a small number of teeth, and they do not have tooth replacement (Butler 1967).
Experimental research on tooth development or odontogenesis is based very largely on the teeth of murine rodents (Butler 1967). Pioneering work by Shirley Glasstone on rat tooth germ cultures gave detailed information about structural, histological, and self-differentiating interactions of explants (Glasstone 1936). However, the dentition of the mouse and that of many other mammals is very different. Mice have highly specialized incisors, distinctive molar patterns, a small number of teeth, and they do not have tooth replacement (Butler 1967).
In vertebrate embryos, a group of cells, called neural crest (NC) cells, separate from the neural tube and migrate away from their parental epithelium to reaggregate with other cells. In the developing embryo, almost all organs, glands, and tissues, such as craniofacial skeleton, cornea, teeth/dentin, thyroid gland, thymus, cardiac septa, adrenal gland, melanocyte, autonomic nerve, sensory nerve, and Schwann cells, have these basic cells (Crane and Trainor 2006; Alberts et al. 2008; Lee et al. 2010a). In addition to the unique invasiveness of NC cells, their contribution toward building the head of vertebrates has been considered to be a turning point in the evolution of the vertebrates (Gans and Northcutt 1983).
Although NC cells are of ectodermal origin, it has been suggested to call them “mesectoderm” or “ectomesenchyme” since they undergo “mesenchymalization.” This property is important to discussions regarding mesenchymal stem cells since their origin is mesenchyme, which is derived from the mesodermal germ layer (Le Douarin et al. 2004). On the other hand, along with the cranial skeleton and other tissues of the head and neck, odontoblasts and tooth papillae are derived from mesectoderm or ectomesenchym (Le Douarin et al. 2004). Oral ectomesenchymal and ectodermal inductive interactions are the earliest expressions recorded in vertebrate fossils. These epithelial (oral ectoderm) and mesenchymal (ectomesenchyme/mesectoderm) interactions phylogenetically precede the origin of odontogenesis (Moss 1969).
Teeth share similarities with the other ectodermal organs, such as hair, feathers, scales, beaks, and many exocrine organs, in the placode and bud stage. These similarities disappear by morphogenesis. Initiation of tooth development includes a series of sequential reciprocal inductive molecular interactions between the dental epithelium and the underlying ectomesenchymal cells (Jernvall et al. 2000). While the first signal inducing differentiation comes from the mesenchyme in all ectodermal organs, in tooth development, morphogenetic events are started by the signals from the epithelium (Pispa and Thesleff 2003). Several recombination studies revealed that only the very early first arch epithelium cells (occurring during the 8–11.5th embryonic days (ED) of mouse development) and ectomesenchyme (ED 12) have odontogenic potential (Mina and Kollar 1987; Lemus 1995).
The molecular aspects of tooth development are similar to those in the development of other organs and include epithelial–mesenchymal interactions. A plethora of molecules (approximately 300) are involved in tooth development such as fibroblast growth factors (FGFs), bone morphogenetic proteins (BMPs), sonic hedgehog (SHH), and wingless integrated (WNTs). As mentioned previously, BMP4 acts antagonistically with FGF8 and this interaction plays a role in the periodic patterning (Neubuser et al. 1997). Irma Thesleff’s group from the University of Helsinki has been working on the key features of dental development. Their web site, http://bite-it.helsinki.fi/, includes a wide range of data largely originating in previously published reports and is thus a compilation of the work of the researchers in this field. The web site provide a source for the expressions of growth factors, receptors, signaling molecules, transcription factors, intracellular molecules, extracellular molecules, and plasma membrane molecules during the different stages of tooth development in mice, rats, humans, and other species. The molecular dialogue between oral ectoderm and odontogenic mesenchyme during tooth development is very complicated (Fig. 2.1) (Jernvall and Thesleff 2000). There is a vast knowledge about the signal interchanges that are crucial to control differentiation and morphological changes and spatiotemporal expression of specific genes during teeth formation, yet little is known about the regulation of the signals (i.e., down or up) (Tucker and Sharpe 2004). All of the data show that there is no single gene that is directly connected with ontogenesis or the lack of any specific tooth. Instead, tooth initiation and morphogenesis occur by an orchestration of numerous genetic and epigenetic factors (Jernvall and Thesleff 2000; Koussoulakou et al. 2009). At the same time, most of the developmental defects in teeth usually occur as a result of mutations in genes encoding signaling molecules and transcription factors (Koussoulakou et al. 2009), such as mutations in the PAX9 gene resulting in partial or total anadontia and mutations in RUNX2 causing supernumerary teeth (Peters et al. 1998; Ryoo et al. 2010).
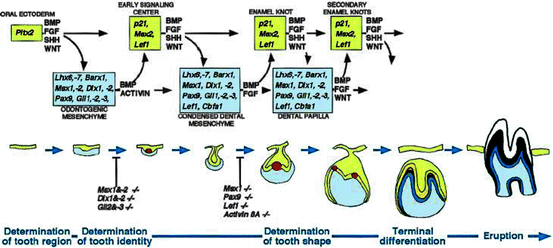

Fig. 2.1
Schematic representation of the signal and transcription factors mediating the reciprocal signaling between epithelium and mesenchyme during advancing tooth development. The molecular cascades are shown above and the corresponding morphological stages below. The transcription factors and signals considered to be important for particular developmental stages are indicated in the squares and above the arrows, respectively. Note how the same signaling pathways are used reiteratively during advancing tooth development, and how tooth development arrests in the knockout mouse experiments to the early signaling center or the enamel knot stage. Key: yellow, tooth epithelium; red, enamel knots; blue, tooth mesenchyme (reproduced from Jernvall and Thesleff 2000 with the permission of the publisher)
2.1 Stages of a Tooth Development
Each tooth passes through four morphological stages: initiation, bud, cap, and cell stages (Fig. 2.2) (Tucker and Sharpe 2004).
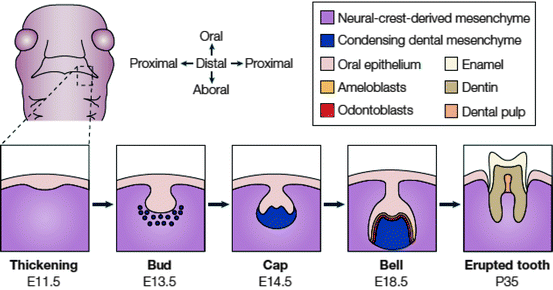

Fig. 2.2
Stages of tooth development. A schematic frontal view of an embryo head at ED 11.5 is shown with a dashed box to indicate the site where the lower (mandibular) molars will form. Below, the stages of tooth development are laid out from the first signs of thickening at ED 11.5 to eruption of the tooth at around 5 weeks after birth. The tooth germ is formed from the oral epithelium and NC-derived mesenchyme. At the bell stage of development, the ameloblasts and odontoblasts form in adjacent layers at the site of interaction between the epithelium and mesenchyme. These layers produce the enamel and dentin of the fully formed tooth (reproduced from Tucker and Sharpe 2004 with the permission of the publisher)
2.1.1 Initiation
The initiation of tooth begins at the end of the fifth week of human gestation and ED 10 of mouse development. A localized thickening or placodes within the primary epithelial bands, formed after about 37 days of development, initiate tooth development. In a subdivision of the primary epithelial band, the dental lamina, localized proliferative activity leads epithelial outgrowths into the ectomesenchyme. Since the underlying ectomesenchyme is more active than the epithelial cells, these ectomesenchymal cells accumulate the epithelial outgrowths soon afterwards. As those cells fold, the forming structure proceeds as per the following descriptive morphological stages of tooth development: bud, cap, and bell. Folding and growth of the epithelium give the final shape of the tooth crown (Fig. 2.2) (Tucker and Sharpe 2004; Nanci 2008).
2.1.2 Bud Stage
This stage occurs between the 7th and 9th weeks of human gestation and ED 11–13.5 in mice embryos. It is represented by the first epithelial invagination into the oral ectomesenchyme. The internal part of the tooth bud contains star-like shaped, glycosaminoglycan synthesizing stellate reticulum cells. Some cells within the stellate reticulum in mice have been identified as putative stem cells (Bluteau et al. 2008). Odontogenic potential is switched from the epithelium to ectomesenchyme during the bud stage. While many ectodermal organs such as exocrine glands, hair follicles, beaks, and teeth share morphological similarity in the bud stage, different ectodermal organs become specific from the beginning of bud-to-cap transition (Jernvall et al. 2000).
2.1.3 Cap Stage
The tooth bud transforms into a cap by differential proliferation and in-folding of the epithelium (Koussoulakou et al. 2009). As the epithelial bud cells proliferate, ectomesenchymal cells condense and morphological differences between tooth germs begin during the cap stage. At ED 12, the histologically distinct epithelial mass, called the enamel knot, is induced by WNT and BMP4. Enamel knot cells do not show cell division and after their transient organizing role is complete, they go apoptosis at the end of the bell stage (ED 16) (Jernvall et al. 1998). Histo-differentiation begins late in the cap stage and in the next bell stage the cells of the crown ameloblasts and odontoblasts are differentiated. A single layer of columnar cells, which border the dental papilla and reside inside the cap, is called inner dental epithelium (IDE). The outer part of the cap is covered by the outer dental epithelium (ODE) (Marson et al. 2008). While the cap-shaped epithelial growth is widely referred to as enamel organ, the condensed ectomesenchymal cells are referred as dental papilla. The dental follicle covers the outside of these two substances. The enamel organ, dental papilla, and dental follicle constitute the tooth germ. The dental papilla is separated from the enamel organ by a basal lamina and is located between IDE and undifferentiated mesenchymal cells of the papilla.
2.1.4 Bell Stage
Terminal differentiation of ameloblasts from IDE and odontoblasts from mesenchymal cells of dental papilla, and the formation of two principal hard tissues of the tooth, enamel, and dentin, are initiated during the bell stage. Ameloblast and odontoblast differentiation is regulated by interactions between the epithelium and mesenchyme (D’Souza 2002; Nanci 2008). While dental papilla is the origin of the future dental pulp, dental follicles give rise to cementoblasts, osteoblasts, and fibroblasts. In conclusion, NC cells give rise to dentin-producing cells, odontoblasts; cementoblasts, which produce root dentin covering; osteoblasts, which participate in the formation of dental alveoli; and fibroblasts, which synthesize collagen for periodontal ligaments (Fig. 2.3).
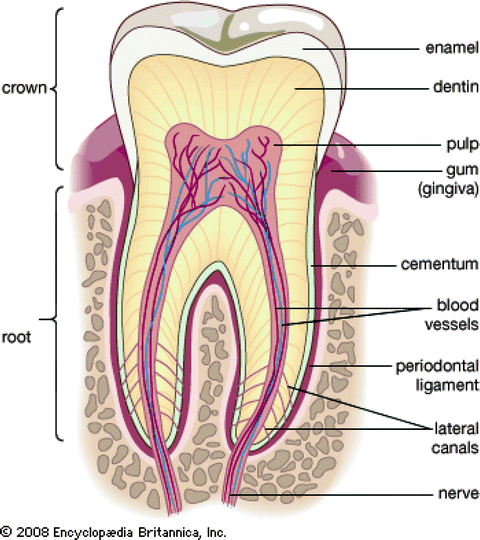

Fig. 2.3
The main structures of the tooth (http://www.britannica.com/EBchecked/media/112882/Cross-section-of-an-adult-human-molar)
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


