(1)
Department of Pediatric Dentistry, Selcuk University, Faculty of Dentistry, Konya, Turkey
Abstract
Generating a pluripotent cell in vitro by rewinding the internal clock of any somatic cell to an embryonic state and then forwarding its conversion into the desired differentiated cell fate represents a rational and ongoing approach in regenerative medicine (Yildirim 2012). There are available human tissues with no ethical or surgical concern, such as fat, blood, biopsy specimens, skin, plugged hair, and extracted teeth (Aasen et al. 2008; Sun et al. 2009; Ye et al. 2009; Yan et al. 2010).
Generating a pluripotent cell in vitro by rewinding the internal clock of any somatic cell to an embryonic state and then forwarding its conversion into the desired differentiated cell fate represents a rational and ongoing approach in regenerative medicine (Yildirim 2012). There are available human tissues with no ethical or surgical concern, such as fat, blood, biopsy specimens, skin, plugged hair, and extracted teeth (Aasen et al. 2008; Sun et al. 2009; Ye et al. 2009; Yan et al. 2010).
DPSC could be effectively reprogrammed into induced pluripotent stem cells (iPSC; Yan et al. 2010; Beltrao-Braga et al. 2011) possibly because they are of mesectodermal origin expressing reprogramming factors inherently (Liu et al. 2011; Yildirim 2012). Our preliminary results clearly indicate that DPSC, SHED, and periodontal ligament stem cells (PDLSC) showing high single-cell clonogenicity responded well to the polycistronic vector, EFα-STEMCCA that includes Oct4, Klf4, Sox2, and cMyc. Using only morphological criteria (Blelloch et al. 2007; Maherali et al. 2007; Meissner et al. 2007) we can say that dental pulp and periodontal ligament-derived iPSC are like embryonic stem cells (ESC): small cells in the colonies with clear borders that separate them from the feeder layer (Fig 7.1). The expression of pluripotency markers of DPSC and SHED has been detected by RT-PCR (Fig 7.2, top). Albeit Sox-2 was not detected before reprogramming, it was consistent through passages in reprogrammed dental cells (Fig 7.2, bottom).
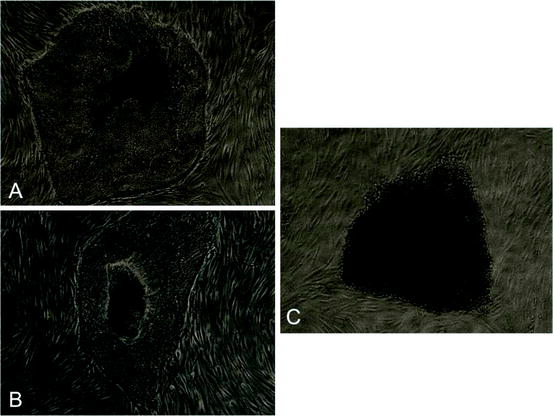
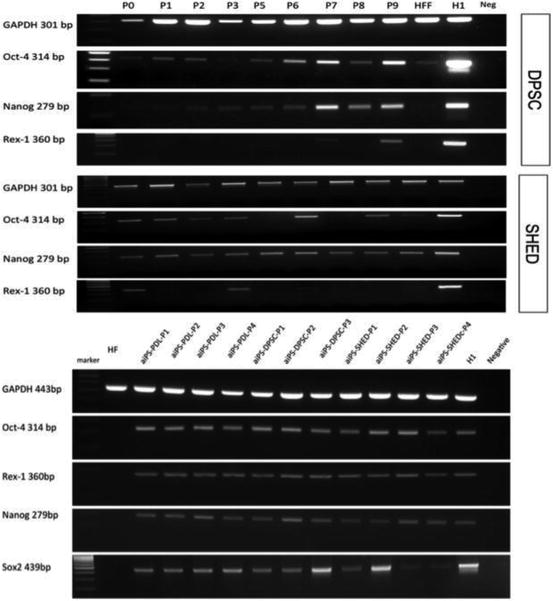

Fig. 7.1
iPSC colonies derived from (a) PDL stem cells, (b) SHED, and (c) DPSC (inverted 100×)

Fig. 7.2
Expressions of pluripotency markers by DPSC and SHED (on top), and derived iPSC (on the bottom) (HFF human fetal fibroblasts, H1 human embryonic stem cell line)
Rapid cycling parental dental pulp and PDL became smaller and continued to grow as monolayer on irradiated human foreskin fibroblast (HFF) feeder layer cells. Although it has not been detected immunocytochemically if most of the cells were expressing the reprogramming factors, the colony forming efficiency was high as they can be induced into the first morphological change of proper reprogramming events (Plath and Lowry 2011). While they were positive for alkaline phosphatase as an intermediate stage marker, continuous expression of the reprogramming factors through early passages may indicate that these cells are good candidates to overcome roadblocks toward pluripotency and to the maintenance of the iPS state, independent of factor expression (Brambrink et al. 2008; Papp and Plath 2011).
It is interesting to note that while SHED- and PDL-derived iPSC colonies have very similar morphologies, iPSC obtained from DPSC display compact dome-shaped morphologies as in mouse ESC. DPSC-derived ones have a different size and shape (Fig 7.2). Since there is no clear information about the differences between permanent and deciduous dental pulp tissues, it is hard to explain why colonies obtained from DPSC and SHED are so different. Yet, it has been proposed that MSC properties of PDL and DPSC are almost identical (Huang et al. 2009), which is in agreement with the morphological similarities of the SHED- and PDL-derived iPSC colonies in this study. The question as to whether those morphological discrepancies reflect epigenetic status of different but closely related cellular origins, from which they arose, deserves more attention.
In the process of reprogramming, as well as the regulation of checkpoints, the genome-wide alteration of epigenetic marks and mesenchymal–epithelial transition (MET) are required for the acquisition of pluripotency (Plath and Lowry 2011). Genetic factors, signaling pathways, and p53 pathways are candidates for reprogramming dynamics (Yildirim 2012). It has been proposed that some (if not all) of the reprogramming factors might be dispensable, when the somatic cells have high expressions of factors (Aoi et al. 2008; Huangfu et al. 2008; Li et al. 2009). Although we have not detected Sox2 expression in our parental cells, Liu et al. have shown Oct4, Sox2, and cMyc, and Kerkis et al. have shown Oct4 and Nanog expressions in DPSC (Kerkis et al. 2006; Liu et al. 2011).
Wnt, TGFβ/Activin/Nodal and BMP signaling have important roles in induced reprogramming (Yildirim 2012). A downstream effector of the Wnt pathway, an inhibitor of glycogen synthase kinase-3 (GSK3), has been shown to maintain pluripotency in human and mouse ESCs (Sato et al. 2004). Moreover, Oct 4 interacts via the Wnt/β-catenin signaling pathway and those events are involved in the maintenance of stem cell integrity and cell fate decision regulation (Abu-Remaileh et al. 2010). Canonical Wnt signals are transduced through Frizzled receptor and LRP5/LRP6 coreceptor to downregulate GSK3, and my RT-PCR screening data on dental pulp tissue gene expression showed that permanent and deciduous dental pulp tissues express LRP5 and Jagged 1 (data not shown), which has been reposted as a β-catenin target gene required for ectopic hair follicle formation in adult epidermis (Estrach et al. 2006).
As one of the most studied pathways, TGF-β/Activin/Nodal plays important roles in the maintenance of the pluripotent state, and tooth development includes almost all members in this pathway (Thesleff et al. 1995). Dental pulp expresses abundantly TGFβ family members (Gu et al. 1996; Sloan et al. 1999; Yildirim et al. 2008).
p53 acts as a barrier to somatic cell reprogramming (Kawamura et al. 2009; Marion et al. 2009; Utikal et al. 2009). It has been suggested that rather than silencing the p53 pathway, selecting target cells with lower levels of p53 and/or higher proliferative ability might give more successful reprogramming (Tapia and Scholer 2010). In dental pulp, p53 could not be detected immunohistochemically (Piattelli et al. 2000).
To minimize or eliminate genetic alterations in the derived iPSC line creation, factor-free human iPSC are necessary. At that rate, dental pulp cells with high proliferation ability and no p53 expression would contribute seamlessly to reprogramming events by the inhibition of TGFβ and BMP signaling (James et al. 2005; Xu et al. 2008), and in addition, Wnt members, such as Wnt3a (Marson et al. 2008), in culture conditions.
7.1 Is Reprogramming Necessary for Dental Regenerative Therapies?
It has been questioned since the emergence of iPSC in cell biology, whether it is necessary to reprogram cells back to the pluripotent stem cell state for regenerative therapies or not. Pluripotency may well not be a prerequisite for the generation of certain differentiated cell types. This groundbreaking discovery of reverting the cellular clock back into the original position may help to dig deeper into cellular differentiation mechanisms. Moreover, modeling many human diseases might be possible by the discovery of cell-type-specific lineage reporters, lineage-tracking tools, and tools to disrupt, repair, or overexpress genes (Saha and Jaenisch 2009). If iPSC technology can provide a step-back system reversing the development, during which there is a gradual loss of differentiative potency proceeding from totipotency to pluripotency and multipotency in committed cell lineages toward terminal differentiation (Hemberger et al. 2009), we would meet with a ground state model that can serve as an observation tower for differentiation and lineage commitment. Since dental pulp has a very heterogeneous cell population including connective tissue cells, unique odontoblasts, immune cells, cementoblasts, and bone cells in a very rich neuronal and vascular environment, pushing ontogenically closed cells back to a common progenitor base (mesectodermal cells/NC cells?) would provide an opportunity for following the pathways through cellular specification. Another possible way to use dental pulp iPSC may be through mimicking epithelial–mesenchymal interactions. Dental pulp contains DPSC, which can differentiate to epithelial cells (Marchionni et al. 2009; Nam and Lee 2009; Sakai et al. 2010; Karaoz et al. 2011; Zhao et al. 2012). After dissociation of epithelial and mesenchymal fractions, these cells can be reprogrammed into dental epithelial-iPSC and dental mesenchymal-iPSC. Since during the early passages of iPSC their epigenetic memories are retained (Kim et al. 2010a, b), these two pluripotent populations can be cocultured with a biomimetic scaffold between them in order to obtain developmental epithelial–mesenchymal interactive events (Fig 7.3).
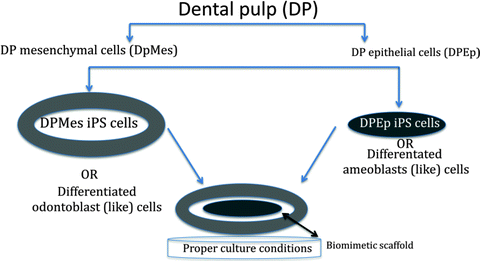

Fig. 7.3
A working hypothesis for epithelial–mesenchymal interactions to mimic dental development
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


