Chapter IV
The Cast Metal-Ceramic Crown
Design
| High gold |
1. Gold-platinum-palladium alloys, |
|
2. Gold-platinum-tantalum alloys, |
|
| Low gold |
3. Gold-palladium-silver alloys, |
| Gold free |
4. Palladium-silver alloys. |
Nickel-chromium alloys;
Cobalt-chromium alloys (rarely used in ceramic bonding).
Typical Formulations
- Gold-platinum-palladium alloys:
Gold 84%, Platinum 7.9%, Palladium 4.6%, Silver 1.3%, Indium and Tin addition approximately 2% (base-metals for bonding); - Gold-platinum-tantalum alloys:
Same as above but palladium replaced by tantalum; - Gold-palladium-silver alloys:
Gold 50%, Palladium 30%, Silver 12%,
Indium and Tin 8% (base-metals for bonding); - Palladium-silver alloys:
Palladium 60%, Silver–balance.
Addition of indium and tin to increase hardness.
Base Metal Alloys
Nickel-chromium:
Ni 68-80%, Cr 11–20% with additions of Mo, Mn, Mg, Al, Si, Be, C, Fe, Ti and Cu (Beryllium is omitted from some alloys). The advantages and disadvantages of each system are summarized in Table 4-1 and certain basic features emerge from a study of this table.
- The higher the gold content the greater the casting accuracy and ease of fabrication.
- Lower palladium content lessens the risk of gas absorption.
- Replacement of palladium by tantalum can produce a more yellow gold coloured ceramic bonding alloy.
- The greater the base-metal content, particularly of nickel and chromium, the greater the problem of controlling oxide production to molecular layers.
- The higher the melting temperature of the alloy the greater resistance to metal creep on firing porcelain.
- 6. High modulus of elasticity and yield strength allows the use of thinner metal sections on longer spans of bridgework.
| Alloy | Advantages | Disadvantages |
| Gold-platinum palladium | Excellent bonding to porcelain. Good castability. Will reproduce fine margins. Easily finished and polished. Corrosion resistant and non-toxic. Yield strength and modulus of elasticity adequate for most types of bridgework. Excellent for reproducing occlusal surfaces. |
Low sag or creep resistance, can distort at fine margins or warp on long span bridges. Yield strength and modulus of elasticity too low for long span bridges unless used in fairly thick section. High cost. |
| Gold-palladium-silver | Higher melting range (ca. 1200°C to 1260°C) giving better creep resistance. Yield strength can be higher than some Au-Pt-Pd alloys. Higher modulus of elasticity, useful in long span bridgework. Good castability. Easily finished and polished. Non-toxic. Low cost. |
Silver content may cause “greening” of porcelain. White colour can show through grey in the mouth. High palladium content can increase risk of hydrogen gas absorption during casting. Bonding to porcelain not yet proven clinically or experimentally. |
| Palladium-silver alloys | High yield strength and modulus of elasticity. Suitable for long span bridges. Non-toxic. Low cost. |
Difficult to cast. Does not reproduce fine margins like the high gold alloys. High silver content can interfere with bonding and cause discolouration of porcelain. High palladium content increases gas absorption. Poor colour. |
| Nickel-chromium alloys | High modulus of elasticity and yield strength allows use in thinner section. Low cost. |
Very difficult to cast accurately. Margins may be short or rougher. Bonding of porcelain and its colour can be affected by excessive oxide production. Permanence of bond yet to be established. Can be toxic in nickel-sensitive patients. Very difficult to remove from teeth in event of repair. |
The only reason for wishing to replace gold-platinum alloys is one of cost and mechanical strength. However, the cost of a gold restoration in the laboratory involves less than one-quarter of the total fee, so that if labour costs are increased the saving in metal is marginal (Smith, 1977). A more sensible approach to the problem of selection of alloys should be one of improved clinical performance since the overall cost to the patient, even assuming gold doubled in price, would still be very low (<8 %).
It is essential to establish in what areas other metal systems can improve the clinical performance of metal-ceramic restorations. There is little doubt that improvements in creep resistance, yield strength and modulus of elasticity are desirable in the gold-platinum alloy systems. The gold-palladium-silver or nickel-chromium alloys cannot achieve this goal without sacrificing other desirable properties. In the case of the base-metal alloys, more work has yet to be done in casting accuracy and development of new investments. The low density nickel-chromium alloys need more force during casting to adapt them to fine margins. They also require investments that retain smooth surfaces at high temperature.
Induction casting machines may be effective in standardising casting procedures but they are no guarantee that the alloy will be properly melted. Base-metal alloys differ in emissivity, and induction casting machines equipped with optical pyrometers are prone to make serious errors. Setting the controls of the machine to the alloy manufacturer’s suggested casting temperatures does not always result in satisfactory results. Moffa (1977) has found that even with these sophisticated devices, an empirical approach is necessary to achieve sound castings.
Even assuming the casting problem is overcome, no solution has yet been found to controlling oxide production at the nickel-chromium/porcelain interface. Use of these materials should therefore, be confined to the specialist and research laboratory.
A more promising alternative to the gold-platinum systems may be found in the gold-palladium-silver alloys. These materials have several desirable properties such as higher creep resistance, and modulus of elasticity. However, due to their high palladium content, they present greater problems with regard to gas absorption since palladium is a known hydrogen acceptor. In addition, both dentist and patient must accept the white colour of these alloys. As the gold content is lowered and palladium and silver are added, the yellow colour is lost. Tarnish resistance in vivo can also be another problem, together with possible greening of the porcelain due to silver contamination.
Factors in the Design Reduction of Stress in the Porcelain of the Preparation and Metal Coping
Unfortunately when porcelain is fired onto the metal coping it is a common experience to find that the coping never fits as well after completion of the baking. The cause of this is:
- Metal creep due to the close approximation of melting temperatures of the gold alloys and porcelain (ca. 150°C).
- Differences in thermal expansion and rate of cooling between gold alloys and porcelain.
- Sintering shrinkage of the porcelain.
The labial or buccal margins, where the thickness of metal is limited by aesthetics, is the region most often subject to distortion. The design of the metal coping must therefore take into account all the above factors and achieve the following objectives:
- Reduction of stress in the porcelain.
- Minimising creep of the metal coping during firing of the porcelain.
- Maintaining the marginal fit during firing of the porcelain.
- Optimising the aesthetics of the facial or buccal porcelain.
- Providing adequate strength to resist masticatory forces.
Reduction of Stress in the Porcelain
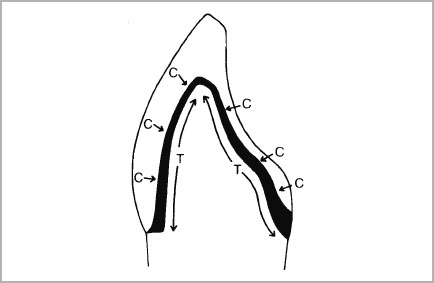
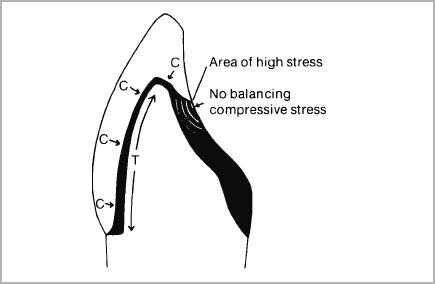
When porcelain is fired onto a rigid metal body, the shape of the metal will influence very considerably the stresses set up in the porcelain. If a coping is designed for full metal coverage, during cooling, the metal being of higher thermal expansion will attempt to contract faster than the porcelain, with the result that the metal is placed in tension and the porcelain in compression (Fig. 4-1). This is an ideal state of affairs. However, when partial metal coverage is used, the porcelain veneer will only cover a limited area of the metal. The junction between the metal and porcelain is therefore a potential site for high stress at the bond since the metal adjoining the porcelain/metal interface will have no balancing compressive stress at the surface (Fig. 4-2).
- The optimum design for a coping should allow full porcelain coverage of the metal so that all tensile stresses developing in the metal on cooling are equally balanced by compressive stresses in the porcelain.
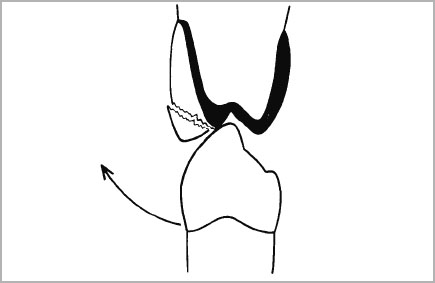
- At no time should opposing cusps contact a junction area between metal and porcelain (Fig. 4-3).
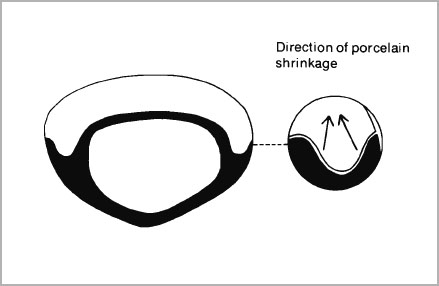
- All metal surfaces for porcelain attachment must be well rounded and present smooth convex outlines. Deep recesses or re-entrant angles must be eliminated since porcelain will tend to shrink away from concavities (Fig. 4-4).
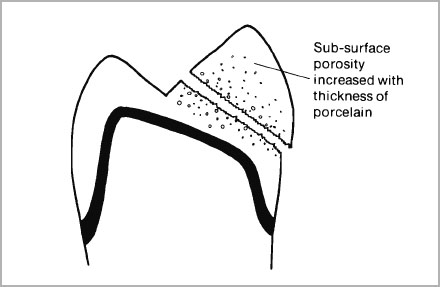
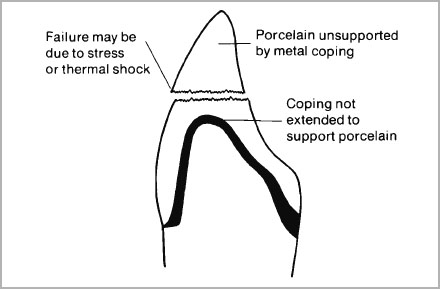
- Metal copings must fully support the porcelain and wherever possible provide even thicknesses of enamel porcelain. Never economise on metal by replacing areas of gross destruction of tooth structure with porcelain (Fig. 4-5).
Figure 4-5b
Fig. 4-5a and b (a) Porcelain which is left unsupported by metal will be more subject to fracture since sub-surface porosity may increase in thicker section. (b) If incisal edges are left unsupported they not only can incur more tensile stress but also be liable to increased thermal shock during glazing.
Minimising Creep of the Metal and Maintaining Marginal Fit During Firing of the Porcelain
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses