Fig. 1
A cluster of titanium dioxide nano-tubes [2]
Nanotechnology therefore, involves materials that have a nano-sized topography or are composed of nano-sized materials ranging between 1 and 100 nm [3]. Nano-biotechnology can be described as a hybrid science that has evolved by blending two predominant technologies—nanotechnology and biotechnology. It essentially involves the further improvement and enhancement of the physical and chemical properties of biomaterials for their better and advantageous use in the medical science [4]. Since, bio-molecules are present in nanometer dimensions, nano-sized biomaterials are expected to integrate and assimilate well in biomedical devices and procedures [5].
An evaluation of nano-mechanical properties of the surrounding bone by nano-indentation revealed that while both implants exhibited similar bone-to-implant contact, the nano-indentation demonstrated that the tissue quality was significantly enhanced around the hydroxyapatite (HA)-coated implants [6]. Since osteoblasts readily adhere to this novel surface, dental implants coated with TiO2 nanotubes could significantly improve healing following dental implant surgery (Fig. 2) [2].
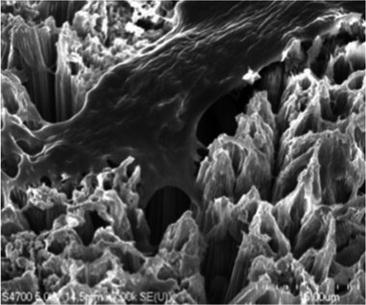

Fig. 2
Bone cell anchoring to a surface of titanium dioxide nano-tubes [2]
Nanotechnology may involve one-dimensional concepts (nanodots and nanowires) or the self-assembly of more complex structures (nanotubes). Materials are also classified according to their form and structure as nano-structures, nano-crystals, nano-coatings, nano-particles, and nano-fibers [3]. Nano-scale modification of the titanium endosseous implant surface may lead to alteration in the topography as well as chemistry of the surface. Therefore, the goal of nanoscale modification should be a specific chemical modification of commercially pure (cp) titanium (grade 4 and 5). A distinct implication associated with nano-scale manipulation of any material is that it also leads to inherent chemical changes on the material surface.
The topographic changes invariably create an increased surface area and nano-scale surface roughness leading to better biological responses of osteogenic cells and effective tissue-implant mechanical interlocking, apart from making the implant surface high wear resistant. These qualities of the nano-biomaterials make it more favourable for implant procedures as compared to other materials. Nano modifications in biomaterials can be undertaken as under:
-
Biomaterial Surface Modifications
-
Alteration of Surface Topography
-
Alteration of Surface Chemistry
-
-
Scaffolds involving Nano Materials
-
Nano-ceramic Composites
-
Nano-fibre Composites
-
These surface modifications enhance solubility and stability of nano materials in aqueous media and make them biologically more compatible. The surface modifications can be achieved by employing physical, chemical and biological methods. Physical modifications can be achieved through molecular coating, molecular adsorption, surface entrapment, plasma spraying and ozone ablation etc. Whereas, chemical modifications may involves procedures like silanisation, surface oxidation and self-assembled monolayers etc.
The advantages of nano-topography based implants or nano-material based implants are significant and may include improved mechanical properties, wear resistance, bone-to-implant contact, protein adsorption, cell response and osseointegration [7, 8].
Dental implants closely mimic natural teeth, but lack in tissue dynamics associated with periodontal tissues. Stem cells offer a promising source for tissue regeneration due to their peculiar quality of proliferation, differentiation and natural plasticity. A positive and desirable interaction of stem cells with various biomaterials offers an enormous scope for their use in various therapeutic applications. Dental stem cells derived from exfoliated deciduous teeth as well as from wisdom teeth are an easy and rich resource for tissue regeneration including osseointegration of implants.
Biomaterials
Biomaterials can be defined as the materials that are well tolerated and accepted when placed inside or adjacent to the living tissues in a body. Biocompatibility therefore, refers to a positive response a material generates in close proximity to the living tissues. The placement of a material in close proximity to the living tissues creates an interface. The material and the tissues come face to face at this interface leading to some reaction or response from both sides. This response is not merely a static reaction but a dynamic one. This reaction or interaction between the material and the living tissues may lead to certain specifically outcomes like:
-
The degeneration of tissues affecting the properties of the material
-
The degeneration of the material affecting health of tissues
-
Regeneration of the tissues and survival of the material
It is this dynamic interaction that determines both—the biological response of the tissues to the material and survival of the material in the biological environment against corrosion and degeneration. That is the basic concept of biocompatibility of a material. Biocompatibility therefore, is not merely a property of the material. Biocompatible activity of a material at the interface of material and the tissue depends on factors like—site of the material placement, its duration in the biological environment, properties of the material and health of the host. Therefore, a material that is biocompatible as a crown and bridge material may not necessarily be compatible as an implant material and vice-versa. Health conditions of the host also make an impact on the biocompatibility response of a material. For that matter, biocompatibility of a young person may be different from that of an older person. The biological response of the same material may vary from a healthy young adult to a young adult affected by some debilitating disease or conditions that hinder or inhibit tissue response.
Therefore, it is important to evaluate and understand the physical, chemical and biological properties of basic components of a material before any biological applications, since these properties determine the success or the failure of an implant material in a long term use.
Broadly speaking, the implant biomaterials can be classified into three groups based on their interaction with the tissues (Table 1).
Table 1
Classification of implant biomaterials
|
Classification
|
Response
|
Effect
|
|---|---|---|
|
Bio-tolerant
|
Formation of thin connective tissue capsules that does not adhere to the implant surface
|
Rejection of the implant, leading to implant failure
|
|
Bio-active
|
Formation of bony tissue around the implant material and strongly integrates with the implant surface
|
Acceptance of the implant leading to success of implant
|
|
Bio-reabsorbable
|
Replaced by the autologous tissue
|
Acceptance of the implant leading to success of implant
|
Bio- tolerant : The term refers to the behavior of a material that depicts minimal interaction with its surrounding tissue when placed in the body. The tissue response may be limited to formation of some connective (scar) tissue at the bone implant interface without any integration to the implant surface.
Bio-active: It refers to a material that actively interacts with the surrounding bone and soft tissues. The bio-active materials may either initiate or promote some regenerative activity once placed adjacent to the living tissues. The tissue response leads to formation of bony tissue at the bone implant interface that strongly integrates with the implant surface. This is the most widely used bio-material category for dental implants.
Bio-resorbable: It refers to that property of a material that upon placement within the human body makes it to gradually dissolve and get replaced by adjacent tissues. The tissue response creates formation of autologous tissue leading to implant success. These types of bio-materials are more frequently used in orthopedic implants.
Desired Properties of Biomaterials
Development of biomaterials is a highly skilled job requiring interdisciplinary collective involvement of professionals like pathologists, clinicians, material scientists, material engineers, biomedical scientists etc. The basic properties that ensure success of an implant adjacent to human tissues can be summarized as—
Mechanical Properties
The mechanical properties of a material play a decisive role in its selection for fabrication of implants. Some of the important properties include hardness, tensile strength, modulus of elasticity and elongation. The response of the material to repeated occlusal loads is dependent on the fatigue strength of the material and determines the long term success of implant under recurring occlusal stresses. The modulus of elasticity of bone varies in magnitude from 4 to 30 Gpa depending on the type of bone and the direction of measurement [9, 10].
The material used for implants should have modulus of elasticity equivalent to that of the bone. Inadequate strength or any mismatch in mechanical properties between implant material and the bone is bound to lead to fracture of implant making it biomechanically incompatible. At the same time, an implant material that has a strength much higher than the bone will prevent transfer of the stress to the adjacent bone leading to bone resorption around the implant and implant failure. The biomechanical incompatibility that leads to death of the bone cells is known as ‘Stress Shielding Effect [11]. Therefore, the material of choice for dental implants has to have a combination of high strength and low modulus of elasticity closer to the bone.
Biocompatibility
The materials used for implants are expected to be well accepted and tolerated by the human tissues without causing any adverse effect or reactions in the body. This quality of a material is known as biocompatibility [12]. Interaction and some reaction start between implant surface and adjacent tissues immediately upon placement of implant in the human body. The reaction involves blood coagulation and adhesion of blood platelets to biomaterial surface and encapsulation of the biomaterial by fibrous tissues.
The biological compatibility of implant materials is directly dependent upon their chemical composition, design of the implant, topographic mechanics and wettability of the implant material. Use of nanotechnology may lead to the development of dental implants with controlled topography and chemistry leading to the development of implant surfaces with predictable and favourable tissue response during osseointegration [13, 14].
High Corrosion and Wear Resistance
The implants should be developed using materials with high wear and corrosion resistance in order to ensure longevity of the dental implant under biological conditions. The longevity of the implant is usually determined by its resistance to abrasion and wear in the mouth. The implant materials having low wear and corrosion resistance release non-compatible metal ions in the body fluids. The released ions cause allergic and toxic reactions in the body [15].
A low resistance to wear leads to formation and deposition of wear debris at the implant-tissue interface causing several chemical and biological reactions in the tissues with ultimate failure of the implant due to its loosening [16].
Osseointegration
Osseointegration is a term founded by Professor Per-Ingvar Brånemark after his important breakthrough in the 1950s when he discovered that bone can integrate with titanium components. Osseointegration has been described as a direct structural and functional bone to implant contact under load [17]. Professor Brånemark named his discovery after Latin word ‘os’ that means bone, and ‘integrate’ that means make whole. When conjoined together it points out to interactive coexistence. It was observed that when a screw-shaped implant fixture made of titanium was carefully placed in the bone, the genetic code that usually makes bone reject a foreign material was not activated. The bone cells were able to attach to the titanium surface resulting in a firm and permanent anchorage for a prosthetic reconstruction. It actually promotes bone regeneration that fills the micro gaps between the implant surface and the adjacent bone. The term has been used to explain the success or failure of the implants in medical and dental science ever since. In the absence of osseointegration, fibrous tissue is formed between the bone and the implant surface. The implant therefore, is not well integrated into the bone resulting in implant loosening and subsequent implant failure [18]. Surface topography, surface roughness, surface material and surface chemistry, all play a significant role for a successful osseointegration.
Impact of Nano Topography in Implants
Titanium and Titanium alloys Ti6Al4V have over the years emerged as the most biocompatible material for dental implants due to their excellent corrosion resistance property. Resistance to corrosion in Titanium occurs due to the formation of biologically inert oxide layer on the surface of the implant body. This oxide layer spontaneously converts into tenacious surface oxide on exposure to air or physiologic saline. This surface oxide transformation leads to the formation of three types of oxides on the implant surface; namely TiO (Anastase), TiO2 (Rutile) and Ti2O3 (Brookite). Of the three oxides, TiO2 (Rutile) is the most stable and the most frequently formed oxide layer that has the potential to regenerate instantly. For that matter, if implant surface gets abraded or scratched during implant placement procedure, this oxide layer can recreate itself spontaneously.
Majority of dental implants today are made of cp titanium (1–4 grades) or titanium alloys like Ti6Al4V. Factors that contribute to the ultimate success of an implant include—
- 1.
Physiological conditions of the patient
- 2.
Implant placement procedure
- 3.
Implant material
- 4.
Implant design, and
- 5.
Implant surface
Albrektsson and Wennerberg [19] divided implant surface quality into three categories—
- 1.
Mechanical properties,
- 2.
Topographic properties, and
- 3.
Physico-chemical properties.
They pointed out that these characteristics are inter-related and a change in any of these groups affects the others as well. This significant observation is quite relevant to the study of nano-surface modifications of the endosseous cp titanium implant surface.
The success of dental implantology to a great extent depends on two basic eventualities. The first is to achieve osseointegration and maintain it through the life of implants. This biological bonding of the implant with the surrounding bone ensures a sound mechanical anchoring in situ. The second is an excellent adaptation and blending of the gingival tissues at the neck of dental implant. This ensures a good sealing of the implant in the soft tissue and isolation of implant body from oral environment and thereby preventing any bacterial lodgment in the region and subsequent peri-implantitis.
This initial tissue response to osseointegration renders primary stability to the dental implant that gradually decreases to pave the way for a secondary tissue response of biological union at the implant tissue interface. The implant material, implant design and implant surface play a very significant and crucial role in the success of secondary tissue response for a lasting osseointegration of the dental implant and may be preceded by a transient phase of decreased implant stability. Surface topography of the dental implants plays a significant role on the quality and quantity of osseointegration post implantation. In order to have favourable osseointegration, the implant surface should promote adsorption of proteins, the initial adhesion followed by differentiation of cells and homogenous tissue integration.
It has been established through studies that Calcium Phosphate (CaP) coatings provide titanium implants with an osteo-conductive surface. The CaP coatings get dissolved in the peri-implant region after implant placement leading to increased ionic strength and blood saturation that in turn initiates precipitation of biological apatite nano-crystals on the implant surface. The inherent proteins in the biological apatite layer promote adhesion of osteo-progenitor cells that generate extracellular matrix of bony tissues [20]. Studies have also revealed that osteoclasts that are capable to resorb the bone cells cause degradation of the CaP coating through enzymatic reactions and create resorption pits on the CaP coated implant surface [21].
CaP particles on the implant surface serve as a catalyst and promote almost immediate osseointegration of implants into the adjacent bone. It is significant to create CaP coating on the implant surface that would disintegrate at a rate similar to apposition of bone so that a direct bone contact may develop on the surface of implant.
A favourable biological interactions between implant and bone interface are greatly dependent upon the topography, chemical compositions and wettability of the implant surface. An implant surface that allows predictable, controlled and guided tissue regeneration is more likely to promote contact osteogenesis [22, 23].
Moreover, nano-surface modifications substantially increase the total surface areas at the interface for osteoapposition leading to better osteointegration of the implant. Introduction of Ti nanotubes (300 nm) resulted in significant increase of up to 3.1 times in the strength of bone-titanium interface in rat femur [22].
Methods of Imparting Nano-Features
General modification in implant materials may be broadly categorized into—physical modifications, chemical modifications and biological modifications and can be summarized as under:
- 1.
Physical Modification
- (a)
Molecular Coating
- (b)
Surface Entrapment
- (c)
Plasma Spraying
- (d)
Ozon Ablation
- (a)
- 2.
Chemical Modification
- (a)
Surface Oxidation
- (b)
PEG (Poly-ethylene-Glycol) Chemistry
- (c)
Silane (Silicone-Based) Chemistry
- (d)
Self-assembled Monolayers
- (a)
- 3.
Biological Modification
- (a)
Antibody–Antigen
- (b)
Receptor–Ligand (Ligand is a molecule like an antibody, hormone or drug that binds to a receptor)
- (c)
DNA–DNA Hybridization
- (a)
There are various methods to create nanoscale features at the implant surface (Table 2) [24].
Table 2
Methods for creating nano-features on cp titanium implants [24]
|
Methods
|
Characteristics
|
|---|---|
|
Self-assembly of monolayers
|
The exposed functional end group could be a molecule with different functions (an osteoinductive or cell adhesive molecule)
|
|
Compaction of nanoparticles
|
Conserves the chemistry of the surface among different topographies. Not readily applied over implant surfaces
|
|
Ion beam deposition
|
Can impart nanofeatures to the surface based on the material used
|
|
Acid etching
|
Combined with other methods (sandblasting and/or peroxidation) can impart nanofeatures to the surface and remove contaminants
|
|
Peroxidation
|
Produces a titania gel layer. Both chemical and topography changes are imparted
|
|
Alkali treatment (NaOH)
|
Produces a sodium titanate gel layer allowing hydroxyapatite deposition. Both chemical and topographic changes are imparted
|
|
Anodization
|
Can impart nanofeatures to the surface creating a new oxide layer (based on the material used)
|
|
Sol–gel (colloidal particle adsorption)
|
Creates a thin-film of controlled chemical characteristics. Atomic-scale interactions display strong physical interactions
|
|
Discrete crystalline deposition
|
Superimposes a nanoscale surface topographical complexity on the surface
|
|
Lithography and contact printing technique
|
Many different shapes and materials can be applied over the surface. Approaches are labor intensive and require considerable development prior to clinical translation and application on implant surface
|
These methods include:
- 1.
Physical methods, like self-assembly of mono-layers, compaction of nano particles and ion beam deposition;
- 2.
Chemical methods, like acid etching, peroxidation, alkali treatment (NaOH) and anodization;
- 3.
Nano particle deposition like sol–gel (colloidal particle deposition) and discrete crystalline deposition; and
- 4.
Lithography and contact printing technique
Influence of Implant Surface on Osseointegration
One of the main concerns related to coating the implant surface is the risk of coating detachment and resultant toxicity of related debris. An evaluation of relationship of particle size and cell viability and proliferation compared to micron-particles revealed that nano-particles of titanium and alumina had less negative impact in cell viability and proliferation as compared to conventional particles. There may be an advantage to nano-scale modification of surfaces using sol–gel coating methods. The quantum interaction of high electron density at the atomic level can enforce high bond strength between the substrate and nano-scale coating [25].
Studies reveal that the addition of a nanometer-scale calcium phosphate treatment to a dual acid-etched implant surface appeared to increase the extent of bone development after 4 and 8 weeks of healing. It was observed that this rapid accrual of bone at the implant surface expedites the implant healing period and supports early loading protocols [26].
Nanotechnology and Cellular Activity
The observation that a micron-scale rough surface prepared by grit blasting and subsequent acid etching was capable of rapid and increased bone accumulation further strengthened an earlier report that a TiO2 grit blasted surface also supported more rapid and increased bone accrual at cp titanium implants [27].
The study also pointed to a significant fact that the cp titanium surface could be modified to enhance bone accumulation and suggested that cp titanium was not only “bioinert” or “biocompatible”, but was also capable to influence cellular activity or tissue responses leading to better and greater osteogenesis and thereby promoting better osseointegration. Nano-topography seems to influence cell interactions at surface of the material being used. It also leads to changed cell behavior when compared to conventional sized topography. The cellular protein adsorption is altered by nanoscale modification of bulk material. Depending on the nano-architecture, cell spreading may be increased or decreased. The present undefined mechanisms indicate that cell proliferation appears to be enhanced by nanoscale topography. Several investigators have shown that nanoscale topography enhances osteoblast differentiation [28–30].
Protein Adsorption (Surface Wettability) in Nano-Surface
Alteration in initial protein surface interaction is believed to control osteoblast adhesion, a critical aspect of the osseointegration process [31]. When implant comes in contact with a biological environment, the initiation of protein adsorption (e.g. plasma fibronectin) promotes subsequent cell attachment and proliferation. Change in surface energy or wettability of a biomaterial corresponds to a typical way of altering cell interactions with the surface. Nano-scale topography is now an established way of altering protein interactions within a surface. An increased vitronectin adsorption has been observed on nano-structured surfaces when compared to conventional surfaces [32, 33]. The study also suggested an increased osteoblast adhesion when compared to other cell types, such as fibroblasts, on the nanosurfaces [33].
Cell Adhesion, Spreading and Motility in Nanosurface
Irrespective of the surface-adsorbed proteins, cells are remarkable in their ability to sense nanostructure. Nano-features of a surface affect both cell adhesion and cell motility. Both of these cell traits are attributed in part, to the function of integrins [33, 34]. Underlying substratum topography influences cell behaviors by both direct and indirect interactions. In 20–40 nm features produced by H2O2/H2SO4 treatment there were definitive interactive points for lamellipodia of spreading cells (Fig. 3) [33]. Cell behavior is affected by both, the dimension and the density of the nano structures.
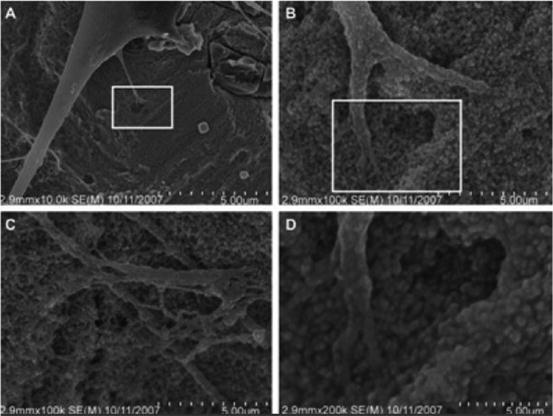

Fig. 3
Nanoscale topography-cell interactions on a nano-surface produced by H2O2/H2SO4. Treatment (a) 10,000 image of adherent cell, (b, c) represent 100,000 images of the same adherent cell and (d) 200,000 magnification of the cell with nano-features. (b) Higher magnification of the rectangle in (a). (d) Higher magnification of the rectangle in (b) [33]
Proliferation in Nano-Surface
Nanosurface modifications promote adherent cell proliferation. Zhao et al., utilizing three distinct methods—electrochemical machining, anodization and chemical etching to produce reproducible submicron-scale structures on titanium surfaces reported an opposing relationship between cell proliferation and cell differentiation with increase in micro scale of surface features.
It has further been supported by studies that observed an increase in the osteoblast proliferation on nano scale materials like alumina, titanium and hydroxyapatite [35, 36]. However, the mechanism involved is still not clear as to how nano-structured surfaces modulate the adherent osteoblast response.
Selectivity of Adhesion in Nano-Surface
Selectivity of cell adhesion is an interesting feature attributed to nanoscale topographic surfaces. Several studies have revealed a relative lowering of fibroblast adhesion compared to osteoblast adhesion on evaluation of nano and micron-structured surfaces [37, 38]. The affinity ratio between osteoblasts and fibroblasts was 3 to 1 on nanosized materials as compared to the conventional materials depicting a ratio of 1 to 1 [33]. Studies on other cell types such as smooth muscle cells and chondrocytes have also reported similar results [39].
All these observations may lead to some major implications in specification of tissue response at bone and mucosal surface of the dental implant/abutment assembly.
Differentiation
A rapid differentiation of adherent mesenchymal cells along the osteoblast lineage is as significant for the process of osseointegration as supporting osteoblast-specific adhesion and adherent cellular proliferation. Studies have revealed that alkaline phosphatase synthesis and calcium mineral content increased in cell layers formed on nano sized materials after 21 and 28 days [40, 41] (Table 3).
Table 3
Summary of the osteochondro-inductive nanostructures presented and the cell behavior outcomes observed [42]
|
Nanostructured materials
|
Fabrication techniques
|
Cell types
|
Cell behaviors
|
|---|---|---|---|
|
TiO2 nanotubes with Ta coating [43]
|
Two electrode setup anodization and vacuum-deposited
|
Osteoblasts
|
Improvement of viability and faster Mineralization
|
|
TiO2 nanotubes [44]
|
Two electrode setup anodization
|
Chondrocytes
|
Promotion of chondrogenesis
|
|
Carbon nanotubes-nanocomposite of CHI fibers + HA crystals [45]
|
Arc discharged method, freeze-drying and lyophilization
Stay updated, free dental videos. Join our Telegram channel
VIDEdental - Online dental courses
 Get VIDEdental app for watching clinical videos
Get VIDEdental app for watching clinical videos

|