Type of hydrogel
Promising features
Experimental approach
Result
References
Peptide-Amphiphile (PA)
• Self-assemble into 3D networks
• Easy to handle
• Can be introduced into small defects
• Cell adhesion sequence RGD and an enzyme-cleavable site had been incorporated to tailor the cell–matrix interactions
• DPSCs had been cultured in PA hydrogels for 4 weeks using osteogenic supplements
• DPSCs differentiated to osteoblast-like phenotype
• Expressed osteoblast marker genes, and deposited mineral
Galler et al. [38]
PEGylated fibrin
• Injectable
• PEGylation decelerates rapid degradation of fibrin
• Constructs of DPSC encapsulated PEGylated fibrin were transplanted in immunocompromised mice for 5 weeks and examined for new tissue formation
• Histologic analysis revealed vascularized soft connective tissue similar to dental pulp with degradation of fibrin and production of a collagenous matrix and mineral deposition
Galler et al. [39]
Self-assembling peptide nanofibers
• Customised for stem cell delivery
• Growth factors can be incorporated
• Ease of synthesis
• Injectable
• Biocompatible
• Biodegradable
• DPSCs had been encapsulated together with fibroblast growth factor basic, transforming growth factor β1, and vascular endothelial growth factor via heparin binding
• The hydrogel within dentin cylinders had been transplanted into immunocompromised mice
• A vascularized soft connective tissue similar to dental pulp was formed
Galler et al. [40]
Galler et al. [30]
Alginate
• Nontoxic
• Biocompatible
• Permeable to small molecular-weight proteins
• Exogenous TGFβ1 loaded alginate hydrogel constructs on human dental pulp repair in vitro
• Dentin matrix secretion
• Odontoblast-like cell differentiation
• Secretion of tubular dentin matrix
Dobie et al. [35]
• DPSCs and HUVECs encapsulated within RGD-bearing alginate framework in 1:1 ratio was supplemented with VEGF and FGF
• The contour of the construct was made to replicate the shape of gutta-percha points
• Both DPSCs and HUVECs showed high cell viability within alginate
• The combination of vascular endothelial growth factor and fibroblast growth factor synergize to significantly up-regulate cell proliferation
Bhoj et al. [36]
Glycol Chitin-based Thermoresponsive (GC-TR) Hydrogel
• Biocompatible
• Injectable
• Conform more easily to the varying contours
• Easy to manipulate as it maintains a sol phase unless the temperature reaches the body temperature
• Effects of GC-TRH on DPSC viability, expression of odontogenic/osteogenic differentiation markers were analysed
• Dentin sialophosphoprotein and dentin matrix protein-1 were expressed by cells cultured in GC-TRS at a higher level than that in cells cultured in collagen
Park et al. [41]
Collagen type I
• Biocompatible
• Radiolabelled rat pulp cells were added to polymerizing type I collagen hydrogel and was implanted in the emptied pulp chamber space in the upper first rat molar
• Labelled cells were then tracked for a period of 3 weeks
• Histological analysis showed mitotically active fibroblastic cells as well as neo-angiogenesis and nervous fibres in pulp equivalents seeded with entire cells
Souron et al. [42]
PEG-fibrinogen (PF)
• Injectable
• Mechanical properties can be tuned by the degree of cross-linking
• The precursor solution can be injected into the dental pulp chamber before rapid gelation by photo-polymerization
• DPSCs were cultured within PF hydrogels with varying degree of cross-linking
• Cell morphology, viability, odontogenic gene expression and mineralization were examined
• DPSCs cultured within the highest cross-linked hydrogel remained mostly rounded in aggregates and demonstrated the greatest enhancement in odontogenic gene expression and mineralization
Lu et al. [43]
Peptide Hydrogel (PuraMatrix)
• Biocompatible
• Injectable
• DPSCs suspended in PuraMatrix was injected into the human tooth slices to verify DPSC differentiation, as measured by expression of DSPP and DMP-1
• After 21 days in tooth slices containing Puramatrix, DPSC cells expressed DMP-1 and DSPP and exhibited cytoplasmic elongations
Cavalcanti et al. [44]
• DPSCs and HUVECs co-encapsulated in PuraMatrix was injected into the roots of human premolars and transplanted subcutaneously in SCID mice
• Formation of pulp-like tissue with higher vasculature and mineralizing capacity in prevascularized group compared with that of DPSC-alone group
Dissanayaka et al. [37]
In cell based regenerative approach, in order for pulp regeneration to occur, the stem/progenitor cells must proliferate and produce new matrix. In addition, there must be stimulation of the odontoblasts to proliferate and produce new dentin. Over the past few years there have been a significant advancement in dentin-pulp regeneration technology by stem cell based approach. Several studies, using small (mouse/rat) animal models, have shown that DPSC encapsulated hydrogel constructs can result in pulp/dentin tissue regeneration partially or completely in the root canals with enlarged apical openings of 0.7–3.0 mm.
Irrespective of the type of target tissue/organ, following implantation, an engineered three-dimensional tissue construct requires to develop rapid vasculature in order to meet the oxygen demand. However, immediately after implantation in vivo, tissue constructs depend solely on the oxygen supply diffuse from the nearest capillary, which could be only up to 200 μm away [32, 33]. If the distance form a capillary exceeds 200 μm, the majority of the cells undergo apoptosis [34]. Therefore, the survival of implanted tissue constructs of greater size requires the formation of a capillary network of its own which can deliver necessary nutrients for the cells. Although host blood vessels start to invade the implanted tissue construct, partly in response to the angiogenic factors secreted by the cells undergoing hypoxia, this process happens very slowly, growing only few tenths of micrometers per day [32]. Use of angiogenic growth factor incorporated hydrogel scaffolds [30, 35, 36] and co-culture of DPSCs with endothelial cells within a hydrogel scaffold [37] are two main approaches that have been investigated to promote rapid vascularization following implantation of a tissue/cellular construct.
In a similar study, we used the peptide hydrogel PuraMatrix as a scaffold system to investigate the role of DPSCs in prompting angiogenesis and the potential for regenerating vascularized pulp in vivo [37]. Human umbilical vein endothelial cells (HUVECs ) , DPSCs, or co-cultures of both cell types were encapsulated in three-dimensional PuraMatrix. The peptide nanofiber microenvironment supported cell survival, cell migration, and capillary network formation in the absence of exogenous growth factors (Fig. 1). Further, we demonstrated that DPSCs enhanced early vascular network formation by facilitating the migration of HUVECs and by increasing vascular endothelial growth factor (VEGF ) expression. Both the DPSC-monoculture and co-culture transplanted groups exhibited vascularized pulp-like tissue with patches of osteodentin after transplantation in immunocompromised mice (Fig. 2). Interestingly, the co-cultured groups showed pulp-like tissues with more extracellular matrix, vascularization, and mineralization than that of the DPSC-monocultures in vivo. Findings of this study highlighted the crucial role of DPSCs in initial angiogenesis. Furthermore, this study convincingly showed the PuraMatrix hydrogel as a promising scaffold with a microenvironment to support cell–cell interactions and cell migration, which contribute to successful dental pulp regeneration.
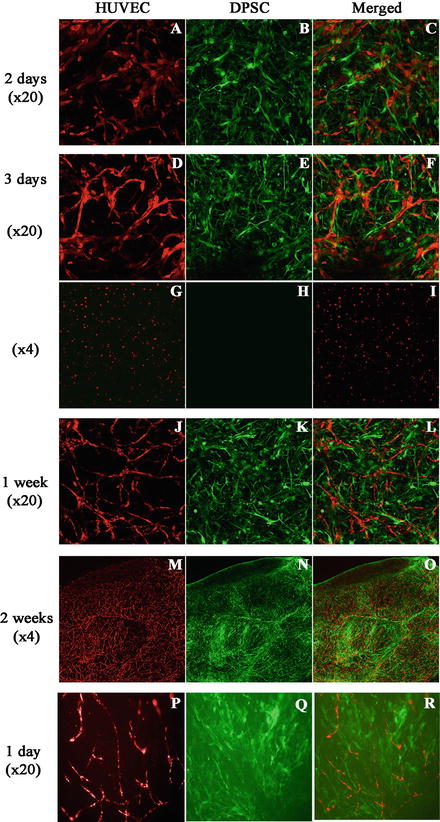
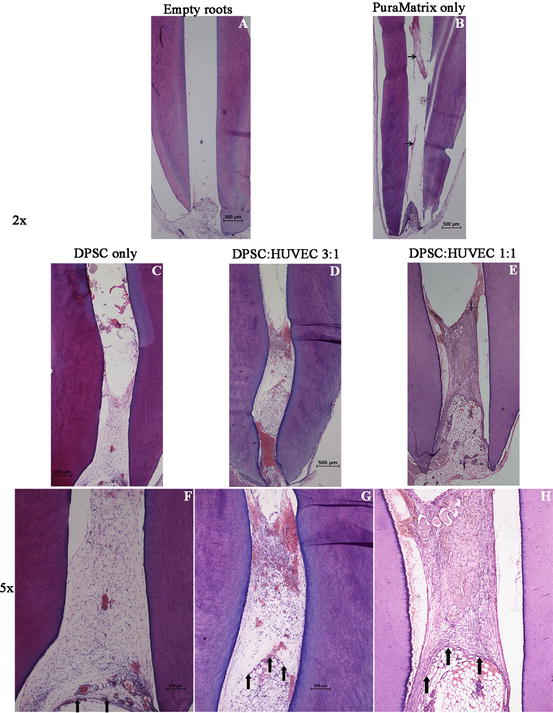

Fig. 1
Vessel-like structure formation by HUVECs that were cocultured as PuraMatrix constructs (Green: green fluorescent protein-expressing cells, Red: red fluorescent protein-expressing cells). (a–c) Coordinated cell migration was observed in the cocultures 48 h after seeding. (d–f) Vessel-like structure formation by HUVECs was observed in the cocultures 3 days after seeding, with DPSCs surrounding the nascent HUVEC networks. (g–i) HUVEC monocultures did not exhibit cell migration or vessel structure formation. (j–o) Vessel-like networks continued to be remodelled and to stabilize for 2 weeks. (p–r) Vascular structure formation was observed after 24 h when cocultured PuraMatrix was injected into root canals of root segments [37]

Fig. 2
Pulp regeneration in cell/PuraMatrix constructs in vivo 4 weeks after transplantation. (a) Empty roots, (b) PuraMatrix-alone in root fragments (black arrows indicate the remnants of PuraMatrix) (c, f) DPSC-alone in PuraMatrix, (d, g) DPSC: HUVEC 3:1 in PuraMatrix, (e, h) DPSC: HUVEC 1:1 in PuraMatrix. Vertical black arrows indicate the border between the transplanted tissue and host tissue [37]
Summary
DPSCs are considered as a promising population of stem/progenitor cells in regenerative medicine for their ready availability, ease of harvesting and stemness properties. Furthermore, DPSCs is the predominant type of stem cells that holds the potential to be used in dental pulp regeneration. Encapsulation of DPSCs within an injectable hydrogel scaffold provides a favourable approach for pulp regeneration as it can be injected to any irregular-shaped defect eliminating the necessity for custom-made scaffold designs. However, regeneration of a pulp-dentin complex that resembles the natural structural organization of cellular elements is yet to be achieved. With regards to this aspect, it is important to develop multiphase hydrogel systems that integrate different cell signalling and differentiation pathways.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


