
5

Sialadenitis
Sialadenitis refers to the various inflammatory conditions affecting the salivary glands. It has been recognized as a disease entity since the time of Hippocrates (c. 460–c. 377 B. C.), who originally delineated a difference between acute suppurative sialadenitis and mumps parotitis.1 Etiologies of sialadenitis include bacterial infections, viral pathogens, granulomatous diseases, and systemic conditions. These inflammatory processes may affect any of the salivary tissues of the head and neck; however, of the major salivary glands, the parotid gland tends to be the most commonly involved site. This predisposition of the parotid gland to inflammation and infection is felt to be due to its anatomy and the physiology of its salivary content and normal salivary flow. Presentation of sialadenitis typically involves diffuse enlargement of the affected gland, accompanied variably by other signs and symptoms of inflammation. The most common presentation, regardless of etiology, is glandular enlargement; however, a broad differential diagnosis should be kept in mind when assessing affected patients. This differential would include noninflammatory conditions, both benign and malignant, which produce swelling and mass effect of the salivary glands, as well as other sources of neck and facial swelling that may imitate sialadenitis. This differential diagnosis is listed in Table 5-1 and includes both salivary and other etiologies of facial and neck enlargement. It is the intention of this chapter to thoroughly delineate the infectious and inflammatory diseases that affect the salivary glands and distinguish these from the other sources of salivary gland enlargement that can complicate diagnosis.
 Acute Suppurative Sialadenitis
Acute Suppurative Sialadenitis
Historically, acute suppurative sialadenitis is a disease of elderly and debilitated patients; it has often been reported in postsurgical patients, particularly after gastrointestinal procedures, which has earned it the nickname “surgical parotitis” or “surgical mumps.”2 It is also a common problem in critical care settings where patients are often kept NPO (nothing by mouth), are unable to maintain their own oral hygiene, and are prone to dehydration and immunosuppression secondary to their chronic illnesses and the treatments thereof. With improvements in modern critical care medicine, including improved antibiotics and vigorous hydration schemes, acute suppurative sialadenitis is a less common entity today.3,4 Still, in the inpatient setting, it reportedly occurs in 0.002 to 0.04% of all postoperative patients. Overall, sialadenitis accounts for 0.01 to 0.02% of all hospital admissions.5 The most recent quoted data found that acute suppurative sialadenitis accounts for 0.03% of hospital admissions in the United States, and 30 to 40% of these cases occur in postsurgical patients.6 In the outpatient setting, a more commonly encountered form of acute sialadenitis is that which is consequent to sialolithiasis. Although the classic postsurgical sialadenitis most frequently involves the parotid gland, stone-related sialadenitis is more commonly noted in the submandibular gland, as it is more prone to sialolith formation3,4 (see Chapters 6 and 7).
The common pathophysiology of acute sialadenitis, be it postsurgical parotitis or stone-related submandibular sialadenitis, is salivary stasis. Stasis of saliva in the ducts and parenchyma may result from poor production of saliva, from impaired salivary outflow, or from a combination of the two. Decreased quantity of saliva is often the result of a state of dehydration, which may be secondary to poor oral intake, overzealous use of diuretics, blood loss, or inadequate fluid replacement in the acutely ill patient. Inanition and anorexia contribute to poor production and flow of saliva. Normally, the presence of a food stimulus prompts salivary production and the action of mastication produces massaging effects of the ducts to facilitate salivary flow. Use of anticholinergic medications and diuretics result in decreased volume of salivary secretions, and sialolithiasis and ductal pathology contribute to impaired flow of saliva through the ducts and into the oral cavity.6
Table 5-1 Differential Diagnosis of Salivary Gland Enlargement
| Inflammatory Sialadenitis Bacterial Viral Granulomatous Systemic (e. g., Sjögren’s syndrome) Cervical adenitis Dental infection or abscess Buccal, masseteric, or deep neck space infection or abscess Bezold’s abscess or lymphangitis otitis externa |
| Noninflammatory Salivary neoplasm Lymphoma Branchial cleft cyst Sebaceous cyst |
The presence of salivary stasis predisposes the salivary gland to infection. Normal salivary flow effectively washes out the parenchyma and ducts of any pathogen that may be present. When the production and passage of saliva are diminished, stagnant saliva provides a ready medium for bacterial growth. It is thought that bacteria migrate from the oral cavity in a retrograde fashion up the ducts to infect the parenchyma of the gland; therefore, organisms of the normal oral flora are the most common culprits in bacterial sialadenitis.7 Staphylococcus aureus accounts for 53% of bacterial infections of the salivary glands; an additional 31% is due to Streptococcus viridans.3 Other potential pathogens include Streptococcus pyogenes and Streptococcus pneumoniae, gram-negatives such as Haemophilus influenzae and Escherichia coli, and anaerobes. A study of parotid abscess drainage specimens demonstrated the isolation of anaerobic bacteria, particularly Bacteroides and Peptostreptococcus species, from 57% of patient samples, highlighting the importance of these bacteria in sialadenitis infections.8 Poor oral hygiene compounds the risk of developing acute bacterial sialadenitis, as an unclean oral cavity acts as a reservoir for bacterial growth. As with any infectious disease, chronically ill and debilitated (particularly hospitalized) patients are more prone to acute sialadenitis, not only because of their diminished immunocompetency but because they frequently cannot maintain adequate oral hygiene, hydration, and normal mastication, all of which place them at risk for acute sialadenitis.
Although the appropriate quantity and normal egress of saliva are the main factors preventing acute bacterial sialadenitis, the quality of saliva is also a determinant of which glands may be involved. In general, saliva has antimicrobial properties that help to prevent dental decay and maintain a clean and healthy oral cavity, inhabited by normal flora. Normal saliva contains various immunoglobulins, lysozyme, lactoferrin, sialoperoxidases, cystatins, and histatins, all of which contribute to the antibacterial nature of salivary secretions. Lactoferrin has particular activity against gram-negative cell walls and is an antistreptococcal enzyme, and the histatins demonstrate both antibacterial and anticandidal properties. Additionally, saliva contains proteins and glycoproteins that inhibit the attachment of bacteria and other pathogens to oral mucosa and dental surfaces.9,10 Unhealthy or diminished saliva will be deficient in these antimicrobial properties. Parotid saliva is more serous than that of the submandibular gland, which is felt to lend it a lesser bacteriostatic quality. The increased viscosity of the mucinous submandibular saliva traps bacteria, halting their progression to the parenchyma of the gland. This difference in salivary composition may account for the greater predisposition of the parotid gland to infection compared with the submandibular gland. That said, the submandibular gland is more commonly affected by sialolithiasis due to the nature of its saliva; higher mucin content and alkaline pH of submandibular salivary secretions, plus increased levels of calcium and phosphate salts, contribute to stone formation.3,6 Blockage of Wharton’s duct by a calculus clearly can lead to the salivary stasis that may lead to the development of an acute episode of sialadenitis. Indeed, sialolithiasis often presents when obstruction of a salivary duct by a stone produces the clinical manifestations of sialadenitis.2
Acute suppurative sialadenitis presents most often with the rapid onset of unilateral painful swelling of the involved gland (Fig. 5–1). The patient may complain of referred pain to the ear, cheek, jaw, or neck, and may experience fevers, malaise, and trismus. Any history of prolonged illness, surgery, dehydration, and poor oral hygiene should be noted, as should a history of salivary stone disease or salivary duct pathology, such as strictures or stenosis. On physical examination, one notes the presence of an enlarged, indurated, warm gland with overlying erythema and tenderness on palpation. There may be reactive cervical lymphadenopathy and displacement of the pinna due to the mass effect of an inflamed tail of parotid. On examination of the oral cavity, there may be edema of the duct and ductal orifice, and purulent saliva may be expressed from the punctum with bimanual massage (Fig. 5–2). Palpation may also reveal the presence of a ductal calculus.4
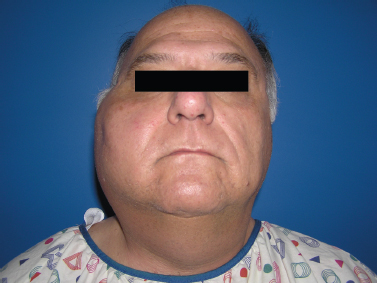
Figure 5-1 Acute parotitis. A patient with significant unilateral parotid swelling that was acute in onset and accompanied by pain on palpation and with mastication.
Diagnosis is typically guided by history and physical examination; however, computed tomography (CT) studies may be undertaken to evaluate for the presence of a nonpalpable stone or abscess formation. One should note that the development of a salivary gland abscess secondary to acute suppurative sialadenitis is typically difficult to detect on physical examination alone, as the dense fibrous capsule of the gland prevents it from feeling fluctuant on exam. Ultrasonography has also been used to evaluate infected salivary glands for the development of abscesses. There are authors who anecdotally report using CT and ultrasound guidance to drain salivary abscesses, but there are no reports comparing these with the standard of surgical drainage.6 Sialography, which involves the injection of contrast dye into the salivary duct orifice, is contraindicated in the setting of acute sialadenitis both because it is of no diagnostic use compared with other imaging modalities and because the contrast agent may cause increased inflammation and flaring of symptoms.4
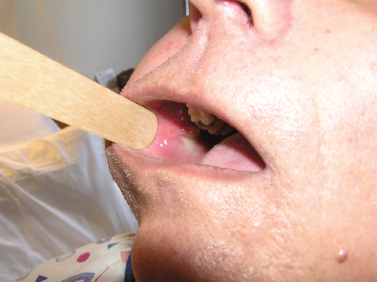
Figure 5-2 Acute parotitis. An intraoral view of the same patient’s Stensen’s duct orifice, which is edematous and expresses purulent saliva with bimanual massage.
Treatment of acute sialadenitis is aimed at eliminating the acute infection and correcting the factors that contributed to the development of the condition. Antibiotic therapy should be administered until clinical improvement is noted, then continued for 1 week after the abatement of symptoms. Although a trial of oral antibiotics is feasible in an otherwise healthy patient, those with underlying exacerbating conditions or with severe constitutional signs should be hospitalized for intravenous therapy.4 A β-lactamase resistance rate of 73% has been demonstrated by parotitis isolates, highlighting the need for appropriate antimicrobial selection; given the preponderance of bacteria that may be involved in a salivary gland infection, broad-spectrum antibiotics are also wise.8 Most authors advocate that a β-lactamase-resistant penicillin or first-generation cephalosporin be used as first-line therapy, given that S. aureus or a Streptococcus species is the most likely pathogen. However, should there be a question whether there is a resistant strain involved (e. g., in a hospitalized patient) or a patient does not improve with standard therapy or is penicillin allergic, other antimicrobials must be used. Vancomycin should be reserved for recalcitrant cases where resistance is suspected. Clindamycin, cefoxitin, imipenem, or a metronidazole-macrolide combination should be used in the case of anaerobic infection or, with the exception of cefoxitin, in penicillin allergic patients.7,8 One should also remember that gram-negative species may play a role in hospital-acquired sialadenitis.11 Pus expressed from the duct may be collected for gram stain and culture to guide further antibiotic choice.
Aggressive hydration is also of great importance to correct dehydration and improve salivary production and flow, thus helping to flush pathogens from the gland parenchyma and clear the infection. Patients should be instructed to drink ample fluids, and treating physicians should have a low threshold for admission for intravenous fluids. Sialagogue use also helps stimulate salivary flow, and warm compresses and analgesics help relieve discomfort. Steroid use may be used to reduce inflammation, particularly of the edematous ductal mucosa, and thus improve ductal drainage during the acute phase. Improvement of any deficiency in oral hygiene should be undertaken, as should daily massage of the gland.
Treatment of sialadenitis that develops secondary to sialolithiasis must address the stone disease as well. Often bimanual massage of the gland will allow one to milk out a smaller stone, or if needed, a small incision in the duct orifice may deliver the calculus. Should the stone be too large for these conservative measures, the acute sialadenitis must be aggressively and adequately treated prior to undertaking any more definitive surgery, such as sialodochoplasty or sialadenectomy for large hilar calculi. Operating on an acutely inflamed gland or duct is technically difficult and may result in postoperative complications of scarring or fistula formation.
Surgical intervention in the setting of acute suppurative sialadenitis may become necessary, however, in the event of abscess formation. As previously mentioned, abscesses of the salivary glands are difficult to diagnose on physical exam. The characteristic fluctuance typical of abscesses is absent due to the tense fibrous capsule of the gland, which envelops the abscess as well. Salivary gland abscess should be suspected in any patient whose sialadenitis fails to improve, or indeed worsens, despite adequate treatment for several days, and prompt CT evaluation is warranted to evaluate for this potentially serious complication. Parotid abscesses can rupture into the external auditory canal or temporomandibular joint. Salivary gland abscesses may also fistulize through the skin of the cheek or neck and extend into the deep spaces of the neck; abscesses originating in the salivary glands have been known to invade the prestyloid parapharyngeal space, the post-styloid space around the carotid sheath, the submandibular space, and the carotid triangle. These abscesses obviously pose a potential threat to the airway, and prompt evaluation and drainage are warranted.3 Surgical drainage of a parotid abscess is demonstrated in Fig. 5–3. This procedure has been in use in essentially the same format since the 1920s. A standard parotidectomy incision is made, and anterosuperior skin flaps are raised. Incisions are made into the parotid fascia parallel to the direction of the facial nerve to liberate the pus, followed by irrigation and closure of the wound over a drain.2,12
Abscess formation is just one of the multiple complications associated with acute suppurative sialadentitis.
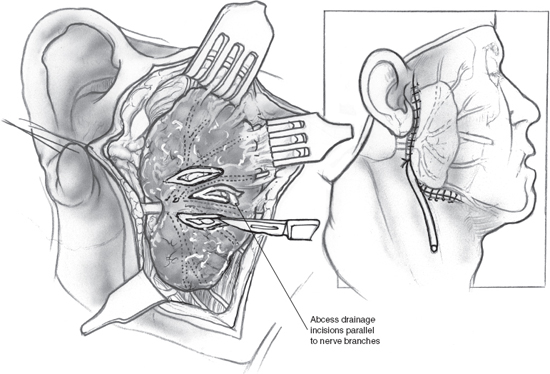
Figure 5-3 Surgical drainage of a parotid abscess. An incision is made as per a parotidectomy. Skin flaps are raised anteriorly and superiorly to reveal the parotid gland with its overlying fat and fascial layers. Incisions are made into the parotid fascia parallel to the course of the facial nerve and its branches, and pus is drained from the abscess cavity. After copious irrigation, the wound is closed over a drain.
Chronic sialadenitis may develop after an episode of severe acute sialadenitis that causes irreversible parenchymal and ductal changes (see discussion later in this chapter). Facial nerve paresis has been rarely reported, and is felt to be due to extreme virulence of the offending organism; the resulting intense inflammation causes compression on the nerve or neuronitis. Given that this is so rare a consequence of acute sialadenitis, one must rule out a more likely condition producing salivary gland enlargement and facial paresis: malignancy. Indeed, compression and obstruction of a duct by salivary gland tumor resulting in sialadenitis have been reported as the presentation of salivary neoplasm.13 Severe and life-threatening complications, though rare, include osteomyelitis, thrombophlebitis of the internal jugular vein, and sepsis.3 Fulminant necrotizing mediastinitis has also been reported as a complication of acute parotitis.14 Severe acute suppurative sialadenitis can be so complicated an infection, and has historically affected an already debilitated patient population, that there has been a 20 to 25% increased morbidity linked to the disease.6 This fact may reflect the poor overall condition and survival of patients who traditionally developed acute suppurative sialadenitis, rather than morbidity due to the sialadenitis itself.
 Chronic Sialadenitis
Chronic Sialadenitis
Often the factors that predispose an individual to an acute episode of bacterial sialadenitis are not easily eliminated, and stasis of salivary secretions becomes chronic. Sialolithiasis may be recalcitrant, ducts may become scarred and stenotic, and sialadenitis may recur either in the acute suppurative form or as a more indolent subacute process. This recurrent and chronic inflammation of the involved salivary gland causes permanent changes in the gland parenchyma and duct system, including sialectasis, duct ectasia and stenosis, and fibrosis of the parenchyma with loss of functional acini. Fig. 5–4 demonstrates an enlarged and fibrosed submandibular gland after sialadenectomy for chronic sialadenitis; on histopathology of this gland, the loss of functional acini and replacement of normal parenchyma with fat and fibrosis are obvious (Fig. 5–5). This progressive inflammatory destruction of both the functional tissue and drainage system of the involved gland results in diminished secretory function of the gland, establishing a state of progressive salivary stasis and predisposing it to further cycles of inflammation. Once again, the parotid gland demonstrates a greater propensity to chronic sialadenitis than the other major salivary glands.
Clinically, chronic sialadenitis is characterized by repeated periods of pain and swelling of a salivary gland separated by asymptomatic intervals of weeks to months. The patient may additionally complain of thickened and/or diminished saliva, reflecting the decreased secretory capability of a progressively fibrotic gland. Often the patient reports a prior history of a severe episode of acute sialadenitis or ductal pathology such as sialolithiasis, stricture, or prior ductal dilation procedures. Indeed, salivary calculi are the most common cause of chronic sialadenitis, accounting for 30% of all cases. Stricture of Stensen’s duct or orifice causes 8% and 4% of cases, respectively, and adjacent scar causes 2% and tumor compression 3%.15
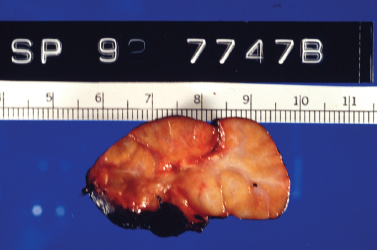
Figure 5-4 Chronic sialadenitis. This resected submandibular gland is enlarged in size and demonstrates a lobular appearance on its cut surface. Note the gray-white fibrotic septae that surround and separate the parenchymal lobules. (Courtesy of Mary Cunane, M. D., Thomas Jefferson University Hospital, Philadelphia, PA.)
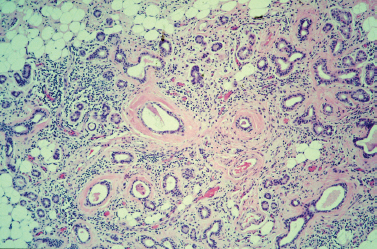
Figure 5-5 Chronic sialadenitis. This photo shows the histologic features of chronic sialadenitis within a submandibular gland. Although the overall lobular architecture is retained, the lobules become atrophic and are separated by fibrous bands. There are prominent infiammatory cell infiltrates throughout the specimen and increased replacement of parenchyma with adipose cells. Note the conspicuous periductal fibrosis and ductal dilation as well. (Courtesy of Mary Cunane, M. D., Thomas Jefferson University Hospital, Philadelphia, PA.)
Treatment of chronic sialadenitis is challenging. It begins with treating the infection and inflammation as one would acute sialadenitis, as chronic sialadenitis is really a complicated and advanced form of that disease. Antibiotics, vigorous hydration, and sialogogues are used, but they do not appear to alter the chronicity of the disease. Any stone disease or other obstructive pathology of the involved duct should be managed appropriately and only after the current inflammation has subsided. A recent review of the literature found that roughly 50% of patients improve or stabilize with these conservative interventions.16 Ultimately, as the disease state progresses and the gland becomes increasingly dysfunctional, conservative treatments fail, and often complete excision of the gland is necessary. Other surgical therapies short of removal of the gland have been attempted, such as injection of the duct with sclerosing agents like methyl violet, duct ligation, and tympanic neurectomy. In theory, these interventions would cause the diseased gland to completely atrophy; however, in practice this outcome is variable in its efficacy. Sialadenectomy is the only definitive solution when medical therapy fails, with the advocated procedure being a near-total parotidectomy in most cases. Delaying surgery until any acute exacerbation has resolved is preferable, as there are conflicting data as to the facial nerve being at higher risk for injury during parotidectomy for chronic sialadentitis than during that for neoplasm.16,17 Preoperative sialography is often advocated to help determine which patients have developed severe enough disease that they might benefit from surgical excision. Those who exhibit moderately severe to severe sialographic changes of irregularly dilated ducts and branches, punctuate sialectasis, and acinar atrophy predictably respond poorly to conservative measures and benefit from surgery. These sialographic changes have been shown to correlate histologically with the severity of the disease.15
 Viral Sialadenitis
Viral Sialadenitis
Although bacterial sialadenitis produces localized infection and inflammation of the involved gland, viral sialadenitis is a manifestation of a systemic infection. Instead of the gland being directly infected by a pathogen as in bacterial disease, it is infected by a hematogenous spread of virus. Mumps is the classic example of viral parotitis. The advent of the human immunodeficiency virus (HIV) epidemic has led to the discovery of HIV-associated salivary gland disease. Other viruses have been known to infect the salivary glands, such as cytomegalovirus, coxsackievirus, echovirus, Epstein-Barr virus, and parainfluenza and influenza strains.18 A discussion of all of these entities is beyond the scope of this chapter; however, one should be aware of the potential of these organisms to cause sialadenitis.
Mumps
Historically, mumps parotitis constituted the most common cause of parotid enlargement, and in the prevaccination era it accounted for its alternate name, “epidemic parotitis.” It was once a common disease of childhood, producing epidemics among children, with a peak age range of 4 to 6 years. It continues to be the most common viral infection of the salivary glands and most frequently affects the parotid gland. Mumps is caused by a paramyxovirus, which is spread by airborne respiratory droplets and is highly contagious. The virus incubates for a period of 2 to 3 weeks prior to the development of clinical signs and symptoms, and viral shedding can occur for 1 week after the onset of symptoms. Clinically, the patient first experiences constitutional symptoms of fever, malaise, and myalgias, which are followed by the development of bilateral parotid swelling and pain that is exacerbated by eating. The other classic manifestation of mumps infection is orchitis, which causes significant discomfort in the acute phase and may be complicated by infertility in the long term. Other potential complications of mumps include encephalitis, pancreatitis, nephritis, and sensorineural hearing loss.
The diagnosis of mumps parotitis is largely clinical, based on the classic presentation of a young patient with bilateral painful parotid swelling and often in the context of an outbreak of mumps in an unvaccinated population. That said, the diagnosis may be confirmed by serologic testing consisting of hemagglutination antigen testing, mumps S and V antigens, and complement fixation testing, or by isolating the virus from urine. As with many viral syndromes, treatment of mumps is largely supportive. Similar to the treatment of other etiologies of sialadenitis, special attention should be paid to keeping the patient well hydrated, not only to support the patient through the systemic viral infection, but also to assist with good saliva production and flow. Comfort measures such as analgesics and warm compresses may additionally be used. One should note that the swelling of the glands, which may be significant, can take weeks to resolve, but it does typically resolve completely. The patient should be followed for chronic sequelae of the infection, including chronic sialadenitis, infertility, and hearing loss.18,19
Human Immunodeficiency Virus
HIV infection may manifest itself as salivary gland enlargement and dysfunction. Salivary gland enlargement in adult HIV patients can also be caused by follicular hyperplasia, viral, bacterial, fungal, and parasitic infections, a Sjögren’s syndrome variant of lymphoepithelial sialadenopathy, diffuse infiltrative CD8 lymphocytosis syndrome, or neoplasms including lymphoma (i. e., mucosa-associated lymphoid tissue lymphoma) and Kaposi’s sarcoma.20
HIV-associated salivary gland disease (HIV-SGD) may be the presenting sign of HIV infection, and it has been noted both in HIV-positive and high-risk HIV-negative populations.20 It is particularly noted in children born to HIV-infected mothers, as well as in adult infected patients. Fifteen to 30% of HIV-infected children demonstrate the bilateral parotitis known as HIV-SGD.21
The lymphoproliferative and cystic changes in HIVSGD ultimately lead to glandular dysfunction.22 It is not known whether the gland becomes infected primarily or if salivary involvement is a manifestation of systemic viral dissemination; we do know that HIV can be found in low levels in saliva of infected individuals, but it is not thought that these concentrations are infective.
HIV infection results in a high concentration and rapid turnover of HIV in hyperplastic lymphoid tissue, in and adjacent to the parotid parenchyma. The fact that the parotid gland as a preferential site for benign lymphoepithelial cysts is probably due to intraparotid lymph nodes, absent in other salivary glands. HIV cytokines and lymphoid hyperplasia stimulate the adjacent ductal epithelium to produce secretions, then secretions cause cyst formation and ductal dilation as well as squamous metaplasia.23
In terms of clinical presentation, HIV-SGD causes gradual nontender swelling of the involved gland, most commonly the parotid glands. Glandular enlargement may initially fluctuate, but eventually it becomes persistent. Benign lymphoepithelial cysts form that are bilateral, multiple, and can become large and disfiguring.24 They can manifest in the superficial or deep parotid lobe and can affect the salivary gland parenchyma and intrasalivary lymph nodes. They are neither invasive nor prone to malignant degeneration.
Along with the classic bilateral parotid enlargement, patients may experience xerostomia, xerophthalmia, and arthralgia, a sicca syndrome not unlike that of Sjögren’s syndrome. It is postulated that this common presentation of HIV-SGD and Sjögren’s syndrome may be related to the fact that both have an autoimmune mechanism to their pathology. Interestingly, glandular biopsy results demonstrate similar inflammatory changes in the gland parenchyma; serologic testing, however, clearly differs between the two disease entities. HIV-positive serology is obviously inherent in the diagnosis of HIV-SGD. Autoantibody SS-A and SS-B titers associated with Sjögren’s are absent in those with HIV-SGD.25
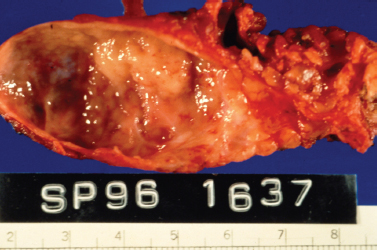
Figure 5-6 HIV-related lymphoepithelial cyst. This is a gross pathology photo of the section surface of a parotid gland lymphoepithelial cyst in an AIDS patient. Note the nodular protrusion of lymphoid tissue into the lumen of the cyst. It was this irregularity that was picked up on imaging and led to the complete resection of the lesion to rule out malignancy. (Courtesy of Mary Cunane, M. D., Thomas Jefferson University Hospital, Philadelphia, PA.)
On gross pathology, the involved salivary gland demonstrates cystic enlargement of the gland and diffuse proliferation of associated lymphoid tissue (Fig. 5–6). Fine-needle aspiration demonstrates a heterogeneous lymphoid population, macrophages, and anucleated squamous cells (Fig. 5–7). The cytology of benign lymphoepithelial cyst in patients with and without HIV infection and with and without Sjögren’s syndrome is indistinguishable.26 When clinical findings and cytology suggest cystic disease, the chance of malignancy is 1%, and nonsurgical management can be considered. The incidence of malignancy in a solid mass of the parotid in an HIV patient is nearly 40%.27
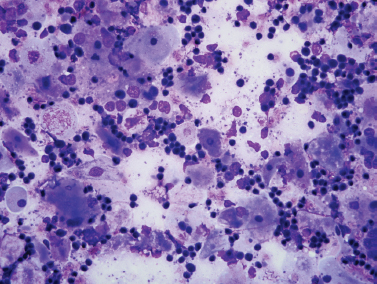
Figure 5-7 Fine-needle aspiration of HIV-associated lymphoepithelial cyst. Smears show a mixed population of benign squamous cells (large, fiat, pale cells) and small benign lymphocytes (Diff-Quik, ×400).
A change in the growth pattern may warrant repeat fine-needle aspiration.
Histopathology reveals multiple epithelial lined cysts of varying size, which arise in the lymphoid tissue of the gland. The cysts are filled with thin yellow fluid containing lymphocytes, macrophages, and cholesterol crystals. Prominent germinal centers are also noted in the lymphoid tissue surrounding the cysts (Fig. 5–8).25,28
When diagnosing HIV-SGD, one must bear in mind that the presentation is essentially of painless salivary gland enlargement. Even if an associated diagnosis of HIV is suspected or known, one must work up the salivary gland lesion as a salivary gland mass and keep in mind that differential diagnosis includes the possibility of neoplasm.
Imaging studies, such as CT and magnetic resonance imaging (MRI), may be helpful to determine the extent and nature of the lesion, as well as to look for a soft tissue component or any associated lymphadenopathy (see Chapter 2, Fig. 2–17). The lymphoepithelial cysts characteristic of HIV-SGD may be seen on such imaging and guide diagnosis. Cervical adenopathy and nasopharyngeal swelling on clinical examination and imaging can distinguish HIV-positive patients with multiple large cysts from solitary lymphoepithelial cysts in patients without HIV.
Tissue biopsy is needed to rule out malignancy, and fine-needle aspiration and possibly even surgical removal of the gland for definitive diagnosis should be considered. Indeed, the latter is still considered the gold standard for diagnosis of HIV-SGD. That said, in the context of known HIV infection with a typical presentation, confirmatory imaging studies, and a negative fine-needle aspiration for neoplasm, some advocate following the lesion closely without proceeding to surgical removal immediately.25,29 The fact that HIV-positive patients have an increased incidence of lymphoma (10% greater than the general population) should also cause one to have a low threshold for obtaining a tissue diagnosis.
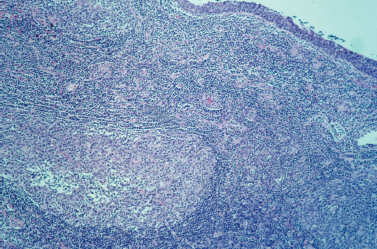
Figure 5-8 HIV-related lymphoepithelial cyst. This low-power view shows the inner epithelial lining of the cyst in the upper right corner, as well as a large lymphoid follicle or germinal center in the lower left corner. Note the predominance of lymphoid cells throughout the specimen. (Courtesy of Mary Cunane, M. D., Thomas Jefferson University Hospital, Philadelphia, PA.)
Conservative treatment of HIV-SGD can be similar to that of other causes of sialadenitis. Adequate hydration is very important, particularly in the context of diminished secretory function and progressive sicca syndrome. Use of sialogogues may help; however, sugar-free sialagogues are preferred, as the diminished flow of saliva predisposes the patient to dental decay. For that very reason, vigorous oral hygiene should be practiced, with use of topical fluoride.
Combination therapy with antiretroviral agents, when associated with undetectable HIV branched-chain deoxyribonucleic acid (DNA) levels and increased CD4 lymphocyte counts, effectively reduces viral replication, lymphoid hyperplasia, and cyst formation.23 Steroids to suppress the inflammatory process underlying HIV-SGD, and the antiretroviral zidovudine have been used to treat the disease.25,30 Medical management supplemented by cyst aspiration for large cysts provides effective control of this problem and improved patient self-esteem. Noncompliant or those with persistent cysts can be managed with fine-needle aspiration or superficial parotidectomy if the patient is stable and a complete informed consent is established.
Radiation therapy with 24 Gray produces durable parotid control for HIV-associated lymphoepithelial lesions, with failures being uncommon after 2-year follow-up.31 The duration of survival continues to improve, and the issue of radiation-induced secondary malignancy requires consideration. Alternatively, doxycycline as a sclerosant is effective, avoiding the risks of surgery and radiation. It is best suited for small cysts in the early stage of development, often as it leaves the patient with a residual parotid mass particularly if the cyst was large at the onset of sclerotherapy.24
 Granulomatous Diseases
Granulomatous Diseases
Multiple granulomatous diseases, both acute and chronic in nature, may affect the salivary glands. Specifically, these diseases affect the lymphatic network associated with the gland, and once again, the parotid gland tends to be more frequently involved compared with the other major salivary glands. Although it is the lymphatic tissue that is predominantly affected, in fulminant disease the inflammatory process infiltrates the adjacent gland parenchyma, impacting its function. These diseases tend to present with gradual enlargement of a nodule within a salivary gland, which is otherwise asymptomatic. As such, one must approach diagnosis as with any solitary salivary gland mass or enlargement, and consider neoplasm, both of the benign and malignant varieties.
Tuberculosis
Involvement of the salivary glands in Mycobacterium tuberculosis infections has been increasingly noted in recent years, reflecting the increased incidence of tuberculosis as a result of the HIV epidemic and new immigrant populations. The salivary glands may reflect either primary infection or secondary or systemic tuberculosis infection. Primary infection of the salivary glands is felt to occur through the intraglandular lymph nodes or via direct infection of the parenchyma. The parotid gland is most commonly involved, and infection typically produces localized glandular disease without constitutional symptoms. Two patterns are noted on histopathology: a diffuse pattern characterized by variably sized nodules occurring throughout the parenchyma, and a localized type consisting of a solitary mass or nodule. Secondary or systemic infection most commonly occurs following primary tuberculosis of the lung and infects the salivary glands via hematogenous spread. It more commonly involves the submandibular and sublingual glands, and the constitutional symptoms characteristic of tuberculosis, fevers, night sweats, and weight loss, are more likely to be present.32
Tuberculosis of the salivary glands initially presents as a rapidly enlarging painless swelling or mass within the involved gland. Due to the lack of concomitant signs and symptoms of inflammation, a differential diagnosis must include salivary neoplasm, lymphoma, and other types of indolent sialadenitis. As the infection progresses, inflammation may become more apparent; the gland becomes tender, and the skin overlying it develops a red or purple hue. The skin may then soften and even rupture, and the resulting sinus tract drain a thin, curdlike fluid.
This clinical picture will lead one to suspect a mycobacterial infection. To confirm diagnosis, a purified protein derivative (PPD) test should be placed, and the salivary mass evaluated with fine-needle aspiration. The fine-needle aspiration is useful in several ways. It may be examined for the presence of acid-fast bacilli (AFB), it provides tissue for AFB culture, and it helps to rule out other causes of salivary gland swelling such as malignancy. The patient should also have sputum cultures taken and have a plain chest radiograph performed to evaluate for cavitary lesions. If diagnosis is not confirmed by these methods, then surgical excision of the gland with histopathologic examination will provide a definitive diagnosis. Inflammation of the parenchyma with the presence of acid-fast bacilli-containing macrophages may be noted on pathology, as are the presence of Langhans’ giant cells. Treatment of tuberculosis infection consists of multidrug regimens, usually consisting of three to four drug combinations taken for 6 to 9 months. Possible agents include rifampin, isoniazid, ethambutol, and pyrazinamide.33,34
Atypical Mycobacteria
Infections of the salivary glands by mycobacteria other than Mycobacterium tuberculosis may present in a fashion similar to that of salivary tuberculosis. There is a multitude of atypical mycobacteria that may produce salivary infection, including M. avium, M. avium-intracellulare, M. malmoense, M. scrofulaceum, and M. bovis. The exact route by which these pathogens infect the salivary glands remains unknown; however, possible conduits of infection include the oral cavity, gingival, lips, tonsils, and throat. Infections are most commonly seen in children 16 to 36 months of age, and both the parotid and submandibular glands have been known to be involved.
The classic presentation is one of a rapidly enlarging salivary gland mass, which develops a violaceous hue to the overlying skin. Progressive disease may become complicated by sinus tracts. Constitutional signs are noticeably absent. A diagnostic work-up similar to that for suspected salivary tuberculosis should be undertaken, given the similarity in clinical presentation. The requisite chest radiograph is negative for the cavitary lesions of tuberculosis, and the PPD is nonreactive. Diagnosis, therefore, is largely clinical and is predicated on the exclusion of other salivary mass entities. Fine-needle aspiration biopsy is preferred to incisional biopsy and will help to rule out other causes of salivary swelling; acid-fast staining is unlikely to be diagnostic, and culturing the tissue is difficult and time-consuming, taking as long as 6 weeks to grow. Incisional biopsy or incision and drainage attempts may facilitate the formation of sinus tracts and unsightly scars. Complete excision of the infected gland is considered to be curative, while there is recent evidence in favor of incision and curettage of the diseased gland. There are no proven effective medical therapies; however, investigations as to the worth of an antibiotic trial are under way. Drugs under consideration include clarithromycin, ethambutol, rifabutin, azithromycin, and fluoroquinolones in adult populations.35
Actinomycosis
Actinomycosis is yet another granulomatous infection of the head and neck that can affect the salivary glands. It is caused by the gram-positive anaerobe actinomyces, which is found in the normal flora of the oral cavity. Within the oral cavity, actinomyces is most prolific in the tonsils and in carious teeth, and infection of the salivary gland occurs via retrograde invasion of the salivary gland duct. Obviously, poor oral hygiene is a key contributor adding to the risk of actinomyces infection; mucosal trauma and disruption, as well as impaired immunity such as that seen in steroid use, diabetes mellitus, and malnutrition, have also been implicated as risk factors. Actinomyces israelii is the most common species and accounts for most infections, but A. bovis and A. naeslundi are also possible culprits.
Actinomycosis of the salivary glands presents as a firm mass within the gland, which may or may not be tender. There is often a history of recent dental work or disease, oral trauma, or oral surgery. The time course of the infection may be rapid and acute, with suppuration much like other causes of sialadenitis, or it may follow an indolent course and produce chronic fibrotic changes within the tissue and the pathognomonic sinus tracts of the disease. These sinus tracts, which are often multiple, drain sulfur granule–containing pus. Other findings on physical exam include induration and erythema of the duct papilla, which may drain purulent saliva. The disease may be complicated by osteomyelitis of the mandible. Constitutional symptoms such as fever or malaise are rare, as is the development of leukocytosis or lymphadenopathy.
Actinomycosis is diagnosed by the presence of sulfur granules and filamentous gram-positive rods on gram stain of swabbed pus or of tissue obtained on fine-needle aspiration. This tissue may also be sent for culture to confirm the diagnosis. If a biopsy of the gland is performed, one will note the presence of multiloculated abscesses surrounded by tough fibrous tissue and containing white-yellow pus. Treatment of actinomycosis consists of a 6-week course of intravenous penicillin G, followed by an additional 6-month oral course of the same. Penicillin G is the antibiotic of choice and produces cure in over 90% of cases. Erythromycin, clindamycin, and doxycycline are also reasonable alternatives. For those cases that prove to be recalcitrant to antibiotic therapy, are suspicious for neoplasm, or if the infection is rapidly progressive and suppurative, surgical excision of the gland is recommended.2
Cat Scratch Disease
The entity known as cat scratch disease is a granulomatous lymphadenitis postulated to be caused by Bartonella henselae, a rickettsial pathogen. Exposure occurs most commonly through domestic cat scratches; 90% of affected persons report some contact with a cat, and 75% recall a scratch or a bite. Canines, however, are causative in 5% of cases.36 The head and neck are the most frequently affected areas of the body, and infection often involves the skin and lymphatics associated with the salivary glands (Fig. 5–9). Clinically, the patient notes a papular lesion that develops at the site of an animal scratch or bite, typically within 2 to 3 days of inoculation. The papule then becomes vesicular and, in some instances, pustular. Within 2 weeks, the proximal lymph nodes enlarge and become tender and erythematous. In more complicated disease, the lymphadenopathy may become suppurative, and encephalitis, arthritis, neuroretinitis, osteomyelitis, or hepatitis may ensue.37
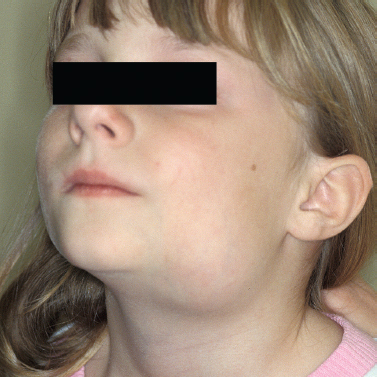
Figure 5-9 Submandibular cat scratch adenopathy.
To diagnose cat scratch disease, three out of the four following criteria must be met: (1) a history of animal (typically feline) contact resulting in a scratch, dermal or ocular abrasion; (2) regional lymphadenopathy occurring within 2 weeks after animal contact, as well as sterile cultures of lymph node aspirate and laboratory results to exclude other causes; (3) a positive cat scratch disease skin test; and (4) a lymph node biopsy demonstrating pathologic features consistent with those of cat scratch disease. Biopsy demonstrates reticular cell hyperplasia, granuloma formation, and widening of arteriolar walls. As the inflammatory process continues, one may observe areas of focal necrosis, which can coalesce to form microabscesses.2 The disease is typically self-limited and requires no specific treatment. Lymphadenopathy should resolve completely within 2 to 4 months; however, aspiration of the node may be of both diagnostic and therapeutic value. Although the disease requires no antimicrobial therapy for complete remission, use of azithromycin has been demonstrated to reduce the duration of lymphadenopathy. Erythromycin, doxycycline, and rifampin have also been used to treat cat scratch disease.38
Toxoplasmosis
The pathogen Toxoplasma gondii is a protozoan parasite, which is most often discussed as a source of congenital abnormalities secondary to maternal infections. Toxoplasma infections have also been more prevalent since the beginning of the acquired immunodeficiency syndrome (AIDS) epidemic; as an opportunistic disease, it affects immunocompromised populations and is an AIDS-defining illness. The organism has a three-phase life cycle, existing as an oocyst in the host cat and as a trophozoite in infected beef, lamb, and chicken. Transmission is through the consumption of these under-cooked or raw meats, or through contact with cat feces. The trophozoite, as the infectious agent, travels to the lymphoreticular system by hematogenous spread, and as a part of the lymphoreticular tissue, the periparotid and intraparotid lymph nodes may become infected. The typical presentation involves isolated neck adenopathy; however, HIV-infected or other immunocompromised patients may develop a disseminated form of the diseases, with myalgias, anorexia, hepatosplenomegaly, pericarditis, or myocarditis. Diagnosis is by isolation of the organism, but acute and convalescent titers may aid diagnosis. Treatment is reserved for severe disease and for pregnant and immune-suppressed individuals. Spiramycin is used in pregnant patients during the first trimester; otherwise, pyrimethamine and sulfadiazine are used in combination.2
Tularemia
The final infectious granulomatous disease to affect the salivary glands is tularemia. It is caused by a gram-negative bacteria, Francisella tularensis, an organism whose host is the cottontail rabbit. Infection occurs when handling infected animals; hunters who skin their rabbits and consumers of rabbit meat are likely patients. The disease may also be transmitted through insect bites (ticks and flies are disease vectors) and even through inhalation. Clinically, an erythematous papule forms at the site of inoculation, which may become nodular and ulcerate. Fever, headache, and tender lymphadenopathy follow 2 to 10 days later. Although the site of lymphadenopathy tends to vary according to the location of the insect bite, the periparotid lymph nodes tend to be affected in head and neck inoculation sites. If the manner of infection was through ingestion of meat or inhalation, then there is no single inoculation site, and lymphadenopathy is diffuse. Diagnosis can be difficult. Patients typically report a history of rabbit handling or tick bite exposure, and serologic testing can be performed. Manipulating the affected lymph nodes in the acute phase for the purposes of obtaining culture or tissue is not recommended for two reasons: The organism is highly difficult to successfully culture, and procedures on the affected nodes may cause dissemination of infection. Streptomycin is the first-line agent for tularemia infection; gentamicin, doxycycline, tetracycline, chloramphenicol, and ciprofloxacin may also be used.39
Sarcoidosis
Sarcoidosis is a systemic granulomatous disease of unknown etiology. It is characterized by the development of noncaseating epithelioid cell granulomas in multiple organ systems. It is more prevalent in African Americans ages 20 to 40 and shows a slight female preponderance. The most common sarcoid presentation is that of bilateral hilar lymphadenopathy, pulmonary infiltrates, the cutaneous lesion erythema nodosum, and ocular manifestations. There are both acute and chronic forms, the former of which responds best to steroid therapy. The most common head and neck manifestation of sarcoidosis is cervical adenopathy, which occurs in 48% of patients.40 More germane to this discussion of sialadenitis is the fact that parotitis occurs in approximately 6% of sarcoid patients, presenting typically as bilateral parotid swelling.41 Indeed, there is a subset of sarcoidosis called Heerfordt’s disease, also known as “uveoparotid fever.” This is characterized by acute parotitis, uveitis or iritis, and fever. Facial nerve paresis has also been rarely reported in conjunction with this syndrome. Typically, Heerfordt’s disease is self-limited, and no treatment is needed; however, other manifestations and exacerbations of sarcoidosis are best treated with corticosteroids and other immune-modulating therapies.39 Rarely, sarcoid involvement of the exocrine glands may present with xerostomia and xerophthalmia. In this case, it may be difficult to distinguish from Sjögren’s syndrome without biopsy of salivary tissue.42
Diagnosis of sarcoidosis relies on biopsy demonstrating the presence of noncaseating granulomas with multinucleated giant cells and epithelioid cells. Minor salivary gland biopsy in patients who have developed signs and symptoms suggestive of sarcoidosis has been proposed in the past as a reasonable and relatively noninvasive way of diagnosing sarcoidosis.43 In the case of salivary sarcoidosis, biopsy of the affected gland, at least via fine-needle aspiration, is necessary to ensure that the glandular swelling does not represent a neoplasm. Sarcoidosis of the salivary gland has been reported to be diagnosed by fine-needle aspiration; the presence of noncaseating epithelioid cell granulomas and multinucleated giant cells is seen on cytology.44,45
 Wegener’s Granulomatosis
Wegener’s Granulomatosis
Necrotizing granulomas and vasculitis are the hallmarks of this idiopathic disease. Involvement of the upper and lower respiratory tract and kidneys is associated with cough, hemoptysis, epistaxis, and constitutional symptoms. The salivary glands are rarely involved and usually in association with systemic manifestations. The presentation is a nondiagnostic pattern of chronic inflammation. CT may demonstrate diffuse salivary gland enlargement. Biopsy and a positive serology for the autoantibody, antineutrophil cytoplasmic autoantibody, cytoplasmic pattern (c-ANCA) are diagnostic. Treatment includes steroids, trimethoprim/sulfamethoxazole, and cyclophosphamides.
 Sjögren’s Syndrome
Sjögren’s Syndrome
The original physician to describe the triad known as Sjögren’s syndrome is of some historical debate. In 1933, Henrik Sjögren, the Swedish ophthalmologist for whom the disease is named, described a syndrome of xeropthalmia, parotid enlargement, and arthritis. It is not clear, however, that he made an association between xerostomia and xerophthalmia from a pathophysiologic standpoint. Johann von Mikulicz Radecki, a Polish surgeon, wrote in 1892 of a link between the two symptoms and noted similar pathologic changes in specimens of lacrimal and salivary gland tissues in patients with these complaints. This led to the eponym Mikulicz disease to describe this disease state. All told, it was not until the mid-20th century that the distinction was made between primary and secondary Sjögren’s syndromes, and that it was a disease of autoimmune mechanism.46
Today Sjögren’s syndrome is described as a sicca syndrome of xerophthalmia or keratoconjunctivitis sicca, xerostomia, and rheumatoid arthritis. It is the second most common rheumatic disease (the most common is rheumatoid arthritis), and two subsets of the syndrome are recognized. Primary Sjögren’s syndrome is characterized by the symptoms of dry eyes and dry mouth without associated rheumatoid disease. Secondary Sjögren’s syndrome is noted to feature the sicca symptoms along with those of another connective tissue disease. Thus the classic xeropthalmia and xerostomia are found in conjunction with rheumatoid arthritis, systemic lupus erythematosus, or scleroderma.47,48 Sjögren’s syndrome affects between 500,000 and 2 million patients in the United States, and it is felt to affect between 1 and 3% of the general population. The disease demonstrates a female preponderance, primarily affecting women between the ages of 40 and 60 years. Although the disease is 9 times more common in women than in men, one should note that it does present in both sexes and in all age groups.46
The common clinical theme of Sjögren’s syndrome is exocrine gland dysfunction. As the immune-mediated destruction of these glands progresses, patients note slowly increasing mucous membrane dryness. The disease is of a chronic and indolent nature, and it is not uncommon for 10 years to pass between the patient’s first notation of mucosal dryness and the diagnosis of Sjögren’s syndrome. From an ophthalmologic standpoint, patients report the sensation of progressively worsening ocular dryness, poor tear production, and red, irritated eyes, often accompanied by foreign body sensation. On examination, conjunctival injection, corneal ulcers, and detached epithelial filaments with discharge may be seen. Scleritis and inflammatory nodules may be seen in severe disease. The examining physician should perform a Schirmer’s test to record the actual impairment of tear production. Five millimeters or less of wetted strip after 5 minutes is considered to be a positive test. Ophthalmologists may additionally perform tear osmolarity and vital dye staining to further objectify tear production and quality in these patients.46–48
Xerostomia is the other major complaint of Sjögren’s syndrome patients. Dry mouth sensation is again a progressively worsening complaint and may be accompanied by a burning sensation of the oral mucosa. Patients may complain of difficulty with deglutition and fluid speech due to their lack of sufficient saliva. Alterations in taste sensation have been reported, as have changes in oral flora and increased incidence of dental caries, the latter reflecting the importance of the bacteriostatic nature of saliva. Enamel decay is noted initially at the gingival and incisive margins.49 Oral examination demonstrates dry-appearing mucosa and a lack of salivary pooling in the floor of the mouth. The tongue may appear smooth with flattening of the filiform papillae, and angular cheilitis may be noted as well.50 One third of affected patients may develop chronic erythematous candidiasis, an atrophic process of the oral mucosa manifested by thin, erythematous patches of mucosa predominantly on the palate and buccal mucosa.46 This may be complicated by candidal thrush superinfection in as many as 80% of patients.47
Salivary gland enlargement (Fig. 5–10) is another common manifestation of Sjögren’s syndrome and may occur in up to one third of patients with the primary form of the disease.49 The parotid gland is most commonly affected, although any major salivary gland may be involved. Enlargement may initially be unilateral and intermittent; however, with time, enlargement of the salivary glands becomes permanent and bilateral. The affected glands are nontender on exam and have a rubbery texture on palpation. The overlying skin may appear tense and erythematous.50
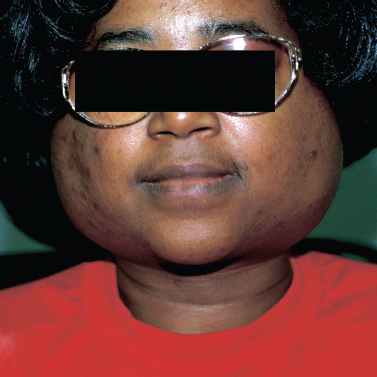
Figure 5-10 Bilateral Sjögren’s syndrome.
Although the clinical signs and symptoms of xerostomia, xerophthalmia, and parotid enlargement may make the physician suspect a diagnosis of Sjögren’s syndrome, quantification of symptoms by various testing measures aids in diagnosis. As previously mentioned, xerophthalmia is commonly diagnosed by the Schirmer’s test. Similarly, xerostomia may be quantified by salivary flow rate testing, salivary scintigraphy, or contrast sialography. On salivary flow rate testing, an abnormal result is considered to be the production of 1.5 mL or less of saliva in a 15-minute period of time; this is considered to be consistent with a diagnosis of xerostomia.48 Nuclear imaging such as salivary scintigraphy may also assist in diagnosis and in following the disease progress. Contrast sialography is not of great value during initial diagnosis of Sjögren’s syndrome, as the pattern of disease is largely indistinguishable from that of chronic sialadenitis; however, it is helpful in following these patients for disease progression and during consideration of possible gland excision. Finally, labial biopsy to examine the minor salivary glands for lymphocytic infiltration and parenchymal fibrosis firmly establishes the diagnosis of Sjögren’s syndrome.51 The upper lip contains the most minor salivary glands (see Chapter 2, Fig. 2–16).
In 2002, a set of diagnostic criteria were developed by the American-European Consensus Group to unify the definition of the disease for both treatment and research purposes. Of the six criteria, one of two mandatory criteria must be met, while a total of four are needed to establish diagnosis of primary Sjögren’s syndrome. The two requisite elements are positive anti-Ro (SS-A) or anti-La (SS-B) serologies [Ro (SS-A) and La (SS-B) are ribonuclear proteins] and a focus score of one or more foci of lymphocytic infiltration on labial minor salivary gland biopsy. A focus is defined as 50 or more lymphocytes per 4 mm2 in the salivary gland tissue. Additional criteria are the presence of ocular symptoms, the presence of oral symptoms, a positive Schirmer’s test, Rose Bengal or ocular dye test, and a positive objective quantification of salivary dysfunction or involvement. The latter includes the aforementioned salivary flow rate testing, salivary scintigraphy, and sialography. Table 5-2 further delineates these criteria. The presence of three out of four of the objective criteria will also classify a case of primary Sjögren’s syndrome. The consensus group also defined exclusion criteria, which include a history of head and neck irradiation, hepatitis C infection, HIV infection or AIDS, preexisting lymphoma, sarcoidosis, graft-versushost disease, and any use of anticholinergic drugs.52
Table 5-2 American-European Consensus Group Classification Criteria for Sjögren’s Syndrome
| Criterion | Description |
|---|---|
| Ocular symptoms | Persistent subjective sense of dry eyes for over 3 months |
| Recurrent foreign body sensation in eye | |
| Use of artificial tears 3 + times daily | |
| Oral symptoms | Daily subjective sense of dry mouth for over 3 months |
| Recent or persistent salivary gland enlargement | |
| Frequent drinking of liquids to assist with swallowing of solid foods | |
| Ocular signs | Positive Schirmer’s test (less than 5 mm in 5 minutes) |
| Rose Bengal/ocular dye score greater than or equal to 4 | |
| Biopsy of minor salivary gland tissue | Biopsy positive for Sjögren’s pathology: focal lymphocytic sialadenitis with a focus score of greater |
| than or equal to 1, where a focus is defined as an area with 50 or more lymphocytes per 4 mm2 | |
| Objective salivary involvement | Unstimulated whole salivary flow less than or equal to 1.5 mL in 15 minutes |
| Parotid sialography demonstrating diffuse sialectasis in the absence of obstructive process | |
| Salivary scintigraphy showing slowed and poor uptake and impaired secretion of nuclear dye | |
| Autoantibodies on serology | Antibodies to Ro (SS-A) and La (SS-B), or both |
Data from Mahoney EJ, Spiegel JH. Sjögren’s disease. Otolaryngol Clin North Am. 2003;36(4):733–745.
There are many extraglandular manifestations of Sjögren’s syndrome that the clinician should be aware of, both within the head and neck and systemically. It is estimated that one third of patients with primary Sjögren’s syndrome experience some extraglandular aspect of their disease. Although a full description of these features is beyond the scope of this chapter, one should note that Sjögren’s patients may experience sensorineural hearing loss, laryngeal lesions, Hashimoto’s thyroiditis and hypothyroidism, and various sinus complaints. There is also a multitude of pulmonary, gastrointestinal, genitourinary, and, of course, musculoskeletal issues that may affect these patients. These associated systemic problems are felt to arise from the same disruption in autoimmune regulation that produces Sjögren’s syndrome.46,50
Of all the associated disease manifestations of Sjögren’s syndrome, the most severe is non-Hodgkin’s lymphoma, which equally affects patients with both the primary and secondary forms of the disease, typically late in its course (see also Chapter 12). The increased risk of lymphoma in these patients is significant; it is cited as 44 times that of the general population and is estimated to occur in 4 to 6% of patients with primary Sjögren’s syndrome.53 Sjögren’s syndrome–related lymphomas are typically marginal-zone B cell lymphomas, and they often present in the organs affected by Sjögren’s syndrome, the parotid and occasionally the submandibular glands. It is postulated that the Sjögren’s syndrome–related lymphomas are the result of the prolonged and chronic stimulation of autoreactive B cells that characterizes Sjögren’s syndrome and that eventually results in their mutation and abnormal proliferation. Sjögren’s patients should be under close surveillance for the development of lymphoma, particularly if they demonstrate any of the following associated risk factors: chronic parotid enlargement, lymphadenopathy, splenomegaly, anemia, lymphopenia, peripheral neuropathy, skin vasculitis, and type II mixed monoclonal cryoglobulinemia.50,53
The pathophysiology of Sjögren’s syndrome is not yet fully understood. It is recognized to have its origins in a disruption of normal immune regulation where exocrine gland tissue is no longer recognized as self and is instead presented as autoantigen. The immune system’s attack response to these autoantigens results in its destruction of exocrine tissue, and hence glandular dysfunction. Exactly what incites this chain of events is unclear, yet several leading theories have been developed. Immune, neuroendocrine, and genetic factors are all felt to contribute to the evolution of the disease, and it is felt that inherent abnormalities in glandular development may be key to the perception of the gland as nonself.
The immune factors that play so elemental a role in the development of Sjögren’s syndrome are felt to originate in defective glandular tissue, which is errantly recognized as nonself antigen by the immune system. Exactly what the defects in the glandular tissues are or what causes them is currently unknown. The presence of these autoantigens in a competent immune system necessarily leads to infiltration of the tissue with lymphocytes and the production of autoantibodies, namely the Ro and La autoantibodies detectable on serologic testing. CD4 T cells appear to be the predominant cell type coordinating the attack, releasing interleukin-1 (IL-1), tumor necrosis factor (TNF), and interferon-γ. This induces other lymphocytes, both T and B cells, to join the attack, with the B cells secreting autoantibodies to further augment the response. This self-perpetuating process leads to the destruction of the majority of the glandular parenchyma in most cases. It should also be noted that the vigorous and chronic nature of this autoimmune response may contribute to the development of lymphoma in Sjögren’s patients. Chronic stimulation of autoreactive B cells may lead to their mutation, resulting in B cell lymphomas. It has also been shown that there is a failure of apoptosis in autoreactive T cells in the Sjögren’s immune response, which may favor the development of malignancy.46
Although the immune destruction of glandular tissue is the pathophysiologic crux of the disease, it cannot fully account for the degree of salivary and lacrimal gland dysfunction we see clinically. The reason for this is that histopathologic examinations of Sjögren’s affected salivary tissue consistently demonstrate destruction of 50 to 60% of acinar and ductal cells, leaving 40 to 50% to continue to secrete.46,54 Some other factors must render the remaining tissue insufficient to maintain appropriate function, thus accounting for the severe xerostomia and xeropthalmia of which patients complain. It is felt that the cytokine release inherent to the autoimmune response of Sjögren’s syndrome impairs normal exocrine activity of the remaining viable cells; TNF and IL-1 have been shown to inhibit neural release of acetylcholine, which is necessary for glandular stimulation. Additionally, antimuscarinic receptor antibodies have been noted in studies using animal models of Sjögren’s syndrome, which would further inhibit normal glandular stimulation.55 Thus these neuroendocrine factors are felt to contribute significantly to the pathogenesis of Sjögren’s syndrome.
Finally, there are notable genetic factors that appear to contribute to the pathogenesis of Sjögren’s syndrome. The most common human leukocyte antigen (HLA) genotype in Sjögren’s syndrome is HLA-DR, with a preponderance of HLA-DR3 among Caucasian patients. There is also evidence that aberrations in glandular development may lead to inherently defective cells that are predisposed to autoimmune recognition.46,50
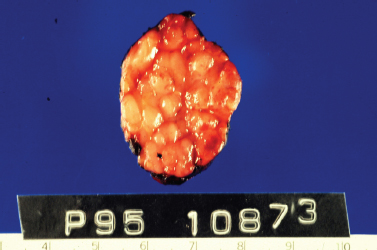
Figure 5-11 Benign lymphoepithelial lesion (BLL) of Sjögren’s syndrome. This submandibular gland is enlarged with nodules of varying size replacing normal parenchyma. (Courtesy of Mary Cunane, M. D., Thomas Jefferson University Hospital, Philadelphia, PA.
Although the diagnosis of Sjögren’s syndrome involves pathologic confirmation by labial minor salivary gland biopsy, the histopathologic pattern of Sjögren’s syndrome in major salivary glands should be noted. The pathologic lesion associated with Sjögren’s syndrome is benign lymphoepithelial lesion (BLL). Grossly, the involved gland demonstrates a nodular pattern of glandular enlargement (Fig. 5–11). Histologically, the affected parenchyma demonstrates lymphocytic infiltration, acinar atrophy, and areas of epithelial proliferation called epimyoepithelial islands (Fig. 5–12). These changes are progressive and may develop into nearly complete replacement of functional parenchyma with lymphoreticular infiltration.56
Treatment of patients with Sjögren’s syndrome continues to focus on symptomatic control rather than disease modulation. Artificial tears for comfort, eye protection, particularly at night, and vigilant ophthalmologic surveillance continue to be the core treatment of Sjögren’s ophthalmalopathy. Meticulous maintenance of good oral hygiene will help to prevent oral and dental complications of the disease. Fluoride treatments and close dental observation with early intervention for dental caries are key elements. Saliva substitutes help with patient comfort, as do sugar-free sialagogues and adequate overall hydration. Oral interferon-αuse is under study, with preliminary data demonstrating improved salivary flow and subjective relief of symptoms. Use of oral muscarinic agents has been shown to improve xerostomia in Sjögren’s patients. Pilocarpine, with its muscarinic receptor agonist mechanism, has been shown to improve salivary secretion, yet it does not appear to have a measurable effect on lacrimal tear production. Cevimeline is a newer muscarinic agonist with a longer half-life than pilocarpine, a higher affinity for M3 muscarinic receptors, and decreased affinity for M2 receptors. The latter reduces the cardiac stimulatory effects of muscarinic therapy.54
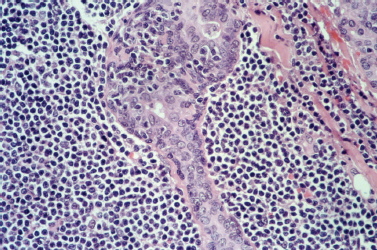
Figure 5-12 BLL of Sjögren’s syndrome. A high-powered view demonstrating an epimyoepithelial island with a tiny lumen surrounded by a mixed population of lymphoid cells. (Courtesy of Mary Cunane, M. D., Thomas Jefferson University Hospital, Philadelphia, PA.)
Treatment of the connective tissue diseases associated with secondary Sjögren’s syndrome has focused on targeting the aberrant immune response responsible for the disease. Steroid therapy in particular is a mainstay of treatment for rheumatoid arthritis and systemic lupus erythematosus, but it has not been shown to affect the sicca complex of Sjögren’s syndrome, either subjectively or objectively. Systemic steroids are used, however, to manage severe extraglandular manifestations of the disease, particularly those of the kidneys and lungs. Antimalarial drugs often used in treating other autoimmune diseases have also been studied as Sjögren’s syndrome therapies with poor results. Thus there are no immune-targeted therapies currently recommended for use in Sjögren’s syndrome.
 Conclusion
Conclusion
The causes of sialadenitis are multiple and diverse, ranging from a multitude of infectious etiologies to the errant autoimmune nature of Sjögren’s syndrome. The common pathologic crux of sialadenitis hinges upon dysfunctional salivary glands. In the case of acute bacterial sialadentitis and mumps, the dysfunction of the gland is transient, lasting long enough to contribute to the pathogenicity of the disease but not becoming a permanent feature of the gland. Whether due to dehydration, sialolithiasis, or simply inflamed parenchyma, the diminished salivary flow of the gland does typically recover with treatment, or even spontaneously. In contrast to this are the sialadenopathies of Sjögren’s syndrome, chronic sialadentitis, HIV-associated salivary gland disease, and sarcoidosis where continued inflammation and replacement of functional glandular tissue cause continued cycles of salivary dysfunction and inflammation. Sialadenitis is really an umbrella term encompassing a wide variety of causative entities, and recognition of this is useful in diagnosing and treating patients with salivary enlargement.
REFERENCES
1. deBurgh Norman, JE. History of the salivary glands and mixed parotid yumor. In: deBurgh Norman, JE McGurk, M, eds. Color Atlas and Text of the Salivary Glands: Diseases, Disorders and Surgery. London: Mosby-Wolfe; 1995: 1–12
2. Gayner, SM Kane, WJ McCaffrey, TV. Infections of the salivary glands. In: Cummings, CW, ed. Otolaryngology Head and Neck Surgery. St. Louis: Mosby; 1998: 1234–1246
3. Raad, II Sabbagh, MF Caransos, GJ. Acute bacterial sialadenitis: a study of 29 cases and review. Rev Infect Dis 1990; 12 (4): 591–601
4. deBurgh Norman, JE. The natural history of lithogenesis and sialolithiasis, acute sialosepsis and sialoadenitis. In: deBurgh Norman, JE McGurk, M, eds. Color Atlas and Text of the Salivary Glands: Diseases, Disorders and Surgery. London: Mosby-Wolfe; 1995: 252–262
5. Perry, RS. Recognition and management of acute suppurative parotitis. Clin Pharm. 1985; 4: 566–571
6. Rice, DH. Non-neoplastic diseases of the salivary glands. In: Paparella, MM, ed. Otolaryngology. Philadelphia: WB Saunders; 1991: 2089–2096
7. Matlow, A Korentager, R Keystone, E Bohnen, J. Parotitis due to anaerobic bacteria. Rev Infect Dis 1988; 10 (2): 420–423
8. Brook, I Frazier, EH Thompson, DH. Aerobic and anaerobic microbiology of acute suppurative parotitis. Laryngoscope 1991; 101: 170–172
9. Ferguson, MM. Salivary gland dysfunction. In: deBurgh Norman, JE McGurk, M, eds. Color Atlas and Text of the Salivary Glands: Diseases, Disorders and Surgery. London: Mosby-Wolfe; 1995: 49–55
10. Ferguson, DB. The physiology and biology of saliva. In: deBurgh Norman, JE McGurk, M, eds. Color Atlas and Text of the Salivary Glands: Diseases, Disorders and Surgery. London: Mosby-Wolfe; 1995: 40–48
11. Pruett, TL Simmons, RL. Nosocomial gram-negative bacillary parotitis. JAMA 1984; 251: 252–253
12. Krippaehne, WW Hunt, TK Dunphy, JE. Acute suppurative parotitis: a study of 161 cases. Ann Surg 1962; 156 (2): 251–257
13. Andrews, JC Abemayor, E Alessi, DM Canalis, RF. Parotitis and facial nerve dysfunction. Arch Otolaryngol Head Neck Surg 1989; 115: 240–242
14. Guardia, SN Cameron, R Phillips, A. Fatal necrotizing mediastinitis secondary to acute suppurative parotitis. J Otolaryngol. 1991; 20 (1): 54–56
15. Zou, Z Wang, SL Zhu, JR Wu, QG Yu, SF. Chronic obstructive parotitis: report of ninety-two cases. Oral Surg Oral Med Oral Pathol 1992; 73 (4): 434–440
16. Motamed, M Laugharne, D Bradley, PJ. Management of chronic parotitis: a review. J Laryngol Otol 2003; 117: 521–526
17. O’Brien, CJ Murrant, NJ. Surgical management of chronic parotitis. Head Neck 1993; 15: 445–449
18. Rice, D. Nonneoplastic diseases of the salivary glands. In: Bailey, BJ, ed. Head and Neck Surgery: Otolaryngology. Philadelphia: Lippincott Williams & Wilkins; 2001: 453–461
19. Van der Waal, I. Inflammatory diseases. In: Diseases of the Salivary Glands: Including Dry Mouth and Sjögren’s Syndrome. New York: Springer-Verlag; 1997: 24–36
20. Wagner, R Tian, H McPherson, M Latham, P Orenstein, J. AIDSassociated infections in salivary glands: autopsy survey of 60 cases. Clin Infect Dis 1996; 22: 369–371
21. de Vries, EJ Kapadia, SB Johnson, JT Bontempo, FA. Salivary gland lymphoproliferative disease in acquired immune disease. Otolaryngol Head Neck Surg 1988; 99: 59–62
22. Oleske, J Minnefor, A Cooper, R, et al. Immune deficiency syndrome in children. JAMA 1983; 249: 2345–2349
23. Craven, D Duncan, R Stram, J, et al. Response of lymphoepithelial parotid cysts to antiretroviral treatment in HIV-infected adults. Ann Intern Med 1998; 128: 455–459
24. Lustig, L Lee, K Murr, A Deschler, D Kingdom, T. Doxycycline sclerosis of benign lymphoepithelial cysts in patients infected with HIV. Laryngoscope 1998; 108: 1199–1205
25. Maynard, JD. Chronic sialopathy and recurrent swellings: sialectasis, Sjögren’s, Mickulicz and HIV-associated salivary gland disease. In: deBurgh Norman, JE McGurk, M, eds. Color Atlas and Text of the Salivary Glands: Diseases, Disorders and Surgery. London: Mosby-Wolfe; 1995: 267–283
26. Maiorano, E Favia, G Viale, G. Lymphoepithelial cysts of salivary glands: an immunohistochemical study of HIV-related and HIVunrelated lesions. Hum Pathol 1998; 29: 260–265
27. Huang, R Pearlman, S Friedman, W Loree, T. Benign cystic vs. solid lesions of the parotid gland in HIV patients. Head Neck 1991; 13: 522–527
28. Ryan, JR Ioachim, HL Marmer, J Loubeau, JM. Acquired immunodeficiency syndrome (AIDS)–related lymphadenopathies presenting in the salivary gland lymph nodes. Arch Otolaryngol 1985; 111: 554–556
29. Falloon, J Eddy, J Wiener, L Pizzo, PA. Human immunodeficiency virus infection in children. J Pediatr 1989; 114: 1–30
30. Schiodt, M Greenspan, D Daniels, TE, et al. Natural history of HIVassociated salivary gland disease. Oral Surg Oral Med Oral Pathol 1992; 74: 326–331
31. Beitler, JJ Smith, RV Brook, A, et al. Benign parotid hypertrophy on +HIV patients: limited late failures after external radiation. Int J Radiat Oncol Biol Phys 1999; 45: 451–455
32. O’Connell, JE George, MK Speculand, B Pahor, AL. Mycobacterial infections of the parotid gland: an unusual case of parotid swelling. J Laryngol Otol 1993; 107 (6): 561–564
33. Painter, DM. Surgical pathology and fine needle aspiration cytopathology. In: deBurgh Norman, JE McGurk, M, eds. Color Atlas and Text of the Salivary Glands: Diseases, Disorders and Surgery. London: Mosby-Wolfe; 1995: 59–89
34. Chaudhary, S. Tuberculosis of the salivary glands. In: deBurgh Norman, JE McGurk, M, eds. Color Atlas and Text of the Salivary Glands: Diseases, Disorders and Surgery. London: Mosby-Wolfe; 1995: 337–339
35. Albright, JT Pransky, SM. Nontuberculosis mycobacterial infections of the head and neck. Pediatr Clin North Am. 2003; 50 (2): 503–514
36. Jackson, LA Perkins, BA Wenger, JD. Cat scratch disease in the United States: an analysis of three national databases. Am J Public Health 1993; 83 (12): 1707–1711
37. Margileth, AM Wear, DJ English, CK. Systemic cat scratch disease: report of 23 patients with prolonged or recurrent severe bacterial infection. J Infect Dis 1987; 155: 390–402
38. Margileth, AM. Antibiotic therapy for cat-scratch disease: clinical study of therapeutic outcome in 268 patients and a review of the literature. Pediatr Infect Dis J 1992; 11: 474–478
39. Choi, E. Tick-borne diseases: tularemia and Q fever. Med Clin North Am 2002; 86 (2): 393–416
40. Schwartzbauer, HR Tami, TA. Ear, nose, and throat manifestations of sarcoidosis. Otolaryngol Clin North Am 2003; 36 (4): 673–684
41. Surattanont, F Mandel, L Wolinsky, B. Bilateral parotid swelling caused by sarcoidosis. J Am Dent Assoc 2002; 133: 738–741
42. Drosos, AA Constantopoulos, SH Psychos, D Stefanou, D Papadimitriou, CS Moutsopoulos, HM. The forgotten cause of sicca complex, sarcoidosis. J Rheumatol 1989; 16 (12): 1548–1551
43. Postma, D Fry, TL Malenbaum, BT. The nose, minor salivary glands, and sarcoidosis. Arch Otolaryngol 1984; 110: 28–30
44. Mair, S Leiman, G Levinsohn, D. Fine needle aspiration cytology of parotid sarcoidosis. Acta Cytol 1989; 33 (2): 169–172
45. Aggarwal, AP Jayaram, G Mandal, AK. Sarcoidosis diagnosed on fine-needle aspiration cytology of salivary glands: a report of three cases. Diagn Cytopathol 1989; 5 (3): 289–292
46. Mahoney, EJ Spiegel, JH. Sjögren’s disease. Otolaryngol Clin North Am 2003; 36 (4): 733–745
47. Rehman, HU. Sjögren’s syndrome. Yonsei Med J 2003; 44 (6): 947–954
48. Jonsson, R Haga, HJ Gordon, TP. Current concepts on diagnosis, autoantibodies and therapy in Sjögren’s syndrome. Scand J Rheumatol 2000; 29: 341–348
49. Daniels, TE. Evaluation, differential diagnosis, and treatment of xerostomia. J Rheumatol Suppl 2000; 61: 6–10
50. Manoussakis, MN Moutsopoulos, HM. Sjögren’s syndrome: current concepts. Adv Intern Med 2001; 47: 191–217
51. Zandbelt, MM Wentink, JR de Wilde, PC, et al. The synergistic value of focus score and IgA% score of sublabial salivary gland biopsy for the accuracy of the diagnosis of Sjögren’s syndrome: a 10-year comparison. Rheumatology (Oxford) 2002; 41 (7): 819–823.
52. Vitali, C Bombardieri, S Jonsson, R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002; 61 (6): 554–558
53. Mariette, X. Lymphomas in patients with Sjögren’s syndrome: review of the literature and physiologic hypothesis. Leuk Lymphoma 1999; 33 (1–2): 93–99
54. Fox, RI Michelson, P. Approaches to the treatment of Sjögren’s syndrome. J Rheumatol Suppl 2000; 61: 15–21
55. Fox, RI Stern, M. Sjögren’s syndrome: mechanisms of pathogenesis involve interaction of immune and neurosecretory systems. Scand J Rheumatol Suppl 2002; 116: 3–13
56. Ellis, GL Auclair, PL. Tumors of the salivary glands. In: Ellis, GL Auclair, PC, eds. Atlas of Tumor Pathology. Washington, DC: Armed Forces Institute of Pathology; 1996: 411–413
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


