Material
Tests
References
Adhesives
Bond strength (shear, torsion, and tensile)
Degree of cure/monomer leaching
Fluoride release/enamel fluoride uptake by adhesives
Critical surface tension
Bonding-induced enamel color alteration
In vivo determination of bonding-induced color alterations in orthodontics
Brackets
Morphology/structure
Torsional stiffness
Hardness
Wear
Roughness
Critical surface tension
Retrieval analysis
Elastomeric modules and chains
Creep and relaxation
Tensile strength
[15]
Fluoride release
The main contribution of the relevant articles to the broader orthodontic materials literature derives from the extended presence of bond strength studies employing a large number of materials and techniques. However, orthodontic polymers as a scientific subject involves also elastomers, which are basically polyurethane modules and chains, latex elastics, and polycarbonate or poly(oxy)methylene plastic brackets.
There is a considerable array of properties of clinical interest, which have attracted investigators: bond strength, degree of cure and fluoride release potential for adhesives; creep and relaxation for elastomeric modules; and hardness, wear, roughness, friction, light transmittance, and torsional stiffness for plastic brackets. Some topics may also be found in associated areas such as metallurgy (for the corresponding properties of interest of stainless steel or titanium brackets, i.e., friction, bond strength, stiffness), and the reader is referred to Chap. 1 for further details.
The purpose of this chapter is to overview the techniques used in these tests and provide a background for the application of these to specific materials.
2.2 Research on Polymeric Adhesives
Research in this area is mainly comprised of bond strength studies, probably because of the apparent simplicity of the experimental setup and the extrapolation of values, which supposedly project the efficacy of the bonding system or the bracket base design.
2.2.1 Bond Strength
Background
A common means of evaluating the potential clinical effectiveness of an adhesive orthodontic material is by measuring its bond strength to a tooth surface. In orthodontics, the bonding of brackets to tooth surfaces is temporary because the attachments are removed after the active treatment period.
Bond strengths are defined as a measure of interfacial adhesion between a substrate and the bonded material, sometimes mediated by an adhesive agent, and are calculated as fracture force divided by the bonded area [1]. In practice, the fractures do not always occur at the interface and often involve the bonded adhesive material and the substrate.
In orthodontic literature, many studies have evaluated the bond strengths in vitro, ex vivo, and in vivo [2]. Most of this research has been under in vitro conditions because it is difficult to expose the materials to and retrieve them from the oral environment without interfering with the environment itself or taxing the subjects’ compliance.
An interesting issue, which has attracted much discussion in the orthodontic materials literature, pertains to the actual clinical requirement of bond strength based on the estimation of the magnitude of forces developed during treatment by the activated archwires. Most studies refer to a 1970s paper by Reynolds and von Fraunhofer [3], which proposed a value of 6–8 MPa. This number has been cited more than 150 times in the relevant literature as the minimum requirement for a clinically derived bond strength threshold value. However, this proposition may not be accurate for the following reasons: (a) It is conjectural since it is based on an assumed profile of the incompletely known load application during mechanotherapy and thus presents an undefined margin of safety. (b) It does not take into account the stresses developed during mastication of hard food or higher chewing velocities. (c) It fails to include aging of the polymeric adhesives and associated environmental stress-fatigue phenomena. Therefore, “threshold strengths” may not cover the requirements for a sound bond throughout the entire period of treatment, which may exceed 18 months.
Description
A variety of teeth have been used in orthodontic bonding experiments, including upper central incisors, premolars, and lower incisors. While premolar extraction may be an integral part of orthodontic therapy, facilitating the collection of those teeth, premolar crown contour variations may complicate the effort to have substrate surface consistency [4]. On the other hand, upper and lower incisors are mostly retrieved from periodontally involved dentitions. Use of such teeth introduces the complicating factor of the age of the average periodontal patient, since the fluoride content in the outermost surface layers has been documented to change with time. Perhaps etching patterns vary accordingly, although no evidence regarding this parameter has been presented [5]. In addition, possible adsorption of inorganic or proteinaceous species, as well as the consequences of various therapeutic procedures and pharmaceutical agents administered to these patients, may modify the reactivity of the enamel surface layers with an undetermined impact on etching patterns. The extraction time and storage media have little if any influence on adhesive bond strength to enamel [6]. This critical review suggests that a storage time of 6 months may be used for normalization purposes among miscellaneous experimental protocols.
Often, experimental treatments of collected teeth include leveling of the prospective enamel surfaces by grinding in an attempt to standardize the topographic variants of the substrate [7]. The argument supporting this notion is related to the incongruities found in the profile contour and convexity of the labial enamel surface, particularly those of premolars. The latter induce a variable pertinent to adaptation of the adhesive layer to the tooth crown, inevitably modifying the composite resin thickness. Although this procedure is obviously inappropriate for clinical conditions, its major flaw is the profound alteration of the substratum. Apparently, surface layers of enamel possess properties dissimilar to those found in deeper zones, due to the higher fluoride content of the outermost 10 μm layer [8]. In addition, grinding of the enamel surfaces is performed ad libitum, using stones or silica disks of varying roughness, while the duration of this process is determined by visual inspection, being thus highly subjective [9]. Therefore, not only is there failure in constructing a simulated clinical analogue, but this method also introduces variability in enamel condition that precludes comparing results from different studies.
The procedure of adhesive application to the bracket base has raised the issues of the quantitative aspects of adhesive and force utilization during bonding. The methods in published studies involve either the application of a standardized quantity of adhesive or the use of an undetermined amount of composite resin [7]. Even though the first approach may normalize variables related to adhesive paste application, allowing for the estimation of reference material properties such as degree of conversion and monomer leaching, it lacks the essential element of simulating the typical clinical procedure employed by orthodontists. A method proposed to overcome this deficiency combines components from both approaches by having multiple pilot trials involving application of an adhesive to bracket bases for bonding by a trained orthodontist [10]. This approach allows an estimate of the weight range of adhesive used, which represents a standardized baseline amount for application to the bracket bases. A similar concern has been expressed about force application during bracket-adhesive attachment to enamel.
Figure 2.1 shows the bond strength cell in which a bracket bonded to the enamel surface of a tooth is placed and subjected to a tensile or shear force to cause bond failure. A value of bond strength is calculated as the quotient of the force at which debonding occurs (determined from the sudden load drop on the mechanical test machine) and the interfacial area of the adhesive or bracket base. Bond strengths have been measured under tensile, shear/peel, and torsional loading, and are reported in units of MPa.
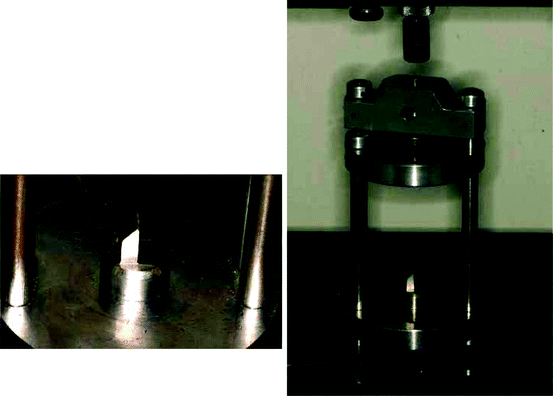

Fig. 2.1
Details of the testing machine cell used for bond strength tests
Limitations
As a rule, bond strength data interpretation should be limited to relative effectiveness of the adhesives included in the study, and thus the tactic of extrapolating absolute values and comparing them with a supposedly clinically derived, “gold standard” should be avoided. This is because the bond strength data derived from a given experiment are associated with the conditions, materials, procedures, and testing variables for this specific experiment and cannot be transferred to a different testing environment.
Although the orthodontic literature is saturated with a wide variety of bond strength papers, considerable variability can be seen among the results for the same materials, probably deriving from the multiplicity of test configurations, as well as the assumptions and approximations integrated in experimental methodologies. For example, it has been demonstrated that the variability among various manufacturers with respect to wing design or dimensions for brackets of a nominally identical prescription may contribute to the misalignment of loads during testing. Thus, the resultant bending moment may vary considerably, which impacts on the stresses developed at the interfaces of the bonding system (enamel adhesive and adhesive bracket). Additional important factors, which may vary among studies, include the method of load application, e.g., tension, shear/peel or torsion, and the crosshead speed of the testing machine, which is typically set to 1 mm/min but may range among studies from 0.5 to 10 mm/min.
The mode of load application and the instrumental configuration for bond strength testing have been discussed by Katona and Chen [11]. Finite element analysis has established that the stress distribution within the adhesive layer and the stresses generated in the brackets and enamel during testing are inhomogeneous, contradicting the uniform stress assumption that has been prevalent in the majority of in vitro experimental protocols. Evidence emphasizing the inappropriateness of comparing results derived from different loading modes (shear, tension, and torsion) was presented, and it was shown that the maximum stresses developed in the orthodontic bonding system under tensile loading may be five times greater than the reported average stress [12]. Hence, traditional bond strength studies substantially underestimate the probability of system failure. Moreover, failure analyses that are intended to provide inferences about the strength of individual components of the bonding system based on their prospective interfacial fracture characteristics should be questioned [13]. This is because the site of failure may arise from crack initiation caused by higher stresses compared with other areas, which is not taken into consideration in the traditional assumption of homogeneous stress.
The validity of comparing results of similar studies is affected by the experimental test configuration, as analyzed by Fox et al. [14]. The applied force may generate moments of various magnitudes, depending upon the distance of the point of force application from the bracket base surface. This parameter may complicate the extrapolation of conclusions regarding the anticipated failure events [15].
The reliability in simulating the strength of appliances during mastication and the occasional contact of brackets with opposing teeth may be questioned, since the standard rates employed in the literature are vastly smaller than the velocity at which teeth occlude during chewing, which may reach 2,000 mm/min [2]. This value greatly affects the risk of bond failure, because very high loading rates eliminate the viscoelastic response of the polymeric adhesive to the applied load, inducing a stiffer elastic response, which decreases bond strength [2].
In addition to the practical problems and standardization difficulties encountered in bond strength testing, a concern has arisen during recent years about the actual clinical relevance of ex vivo bond strength protocols. These studies fail to simulate the multifactorial intraoral aging of resin composites, which include pH fluctuation, complex cyclic loading, microbial attack, and enzymatic degradation [2].
In spite of the presumed appropriateness of simulating the in vivo milieu in laboratory testing, it is worth noting that the oral environment contains a number of parameters which are impossible to reconstruct in an ex vivo model. Some of these factors are the stresses arising from an activated archwire coupled with occlusal loads, extreme pH and temperature variations, and the presence of complex oral microflora and their by-products. This latter factor has been found capable of inducing substantial alterations in the structure and surface properties of orthodontic adhesives [16] and archwires in the oral cavity [17]. In particular, orthodontic adhesive degradation induced by microbial attack during treatment has been recently described by Matasa [16], who examined retrieved brackets intended for recycling.
In summary, some critical aspects of orthodontic bond strength protocols that affect the outcome of research trials may include the following:
1.
The crosshead speed of the loading plate in shear testing is usually set at 0.5 mm/min for consistency, although this value lacks correspondence to clinical conditions. In vivo debonding incidents are expected to occur at much higher impact velocity where viscoelastic behavior of the adhesive, which may be important at low crosshead speeds, is largely absent.
2.
In debonding procedures where the bracket is pulled with the use of a wire loop, the loop harness adaptation and frictional resistance may complicate interpretation of the results. Katona and Chen [11] propose that long, thin wires should be used in such an experimental model.
3.
Bracket design may contribute to misalignment of load application, making the bonding system prone to failure, depending on the stress gradients generated. It has also been found that variability exists among manufacturers with respect to wing design or dimensions for brackets with a nominally identical prescription. This variability poses a substantial problem for the comparison of studies evaluating bracket bond strength.
2.2.2 Degree of Conversion
Background
During the setting process, orthodontic adhesives are polymerized by a free radical mechanism, in which the functional dimethacrylate monomers are converted into a polymer network. However, the in situ polymerization does not always fully convert the monomer to a polymer. It is evident from many studies that all of the dimethacrylate monomers exhibit considerable residual unsaturation of the carbon-carbon double bonds (C=C) in the final product. The degree of conversion (DC) ranges from 45 to 60 % [18]. The unconverted methacrylate groups must reside in the polymer network either as residual monomer or as pendant side chains (in the majority of cases) which extend from the main chains by virtue of having reacted at only one end of the difunctional molecule.
The DC depends upon numerous factors: (a) the structure, composition, type, and polarity of the monomer molecules contained in adhesives; and (b) the exposure time, photoinitiator concentration, light intensity emitted by the curing unit at the peak absorbance wavelength of the photoinitiator, and filler volume fraction in light-cured adhesives [19].
In general, the DC of polymer adhesives modulates the physical and mechanical properties of the material, particularly solubility and degradation. This effect has a pivotal role in altering the biological performance of these materials, since an insufficiently dense polymer network arising from a decreased conversion of (C=C) bonds results in monomer leaching and release of substances, such as plasticizers, polymerization initiators, and inhibitors. Concurrently, the demands for the safety of the patients have strongly been emphasized in various legislations, because of the potential side effects arising from the action of various polymer derivatives to induce allergic, mutagenic, and carcinogenic effects at the cell and tissue levels [20].
Description
FTIR (Fourier transform infrared) spectroscopy has been extensively used to study the degree of C=C conversion in dental dimethacrylate-based resins for various applications (sealants, adhesives, and restoratives) and to evaluate the extent of the acid–base reaction in glass-ionomer materials and their modifications. The basic principles of this technique are based on the fact that when a polymer sample is irradiated by infrared radiation (IR) at photon energies from about 0.05–0.5 eV, the molecules are excited into specific vibrational states due to resonance absorption at frequencies characteristic of the chemical bonds present. Thus, information about the structures of polymeric materials can be obtained. However, this is possible only when changes in dipole moment occur after irradiation. Most studies are performed at the mid IR region of 4,000–400 cm−1.
A major use of FTIR spectroscopy is quantitative analysis. Matching of an unknown spectrum with a reference spectrum is the most positive form of identification. Otherwise band-by-band assignments should be made based on reference databases or custom-built spectral libraries. Subtraction techniques can be employed for the interpretation of spectra, but care should be exercised with internal reflectance and photoacoustic spectroscopy techniques where the depth of beam penetration depends on the wavelength. Several techniques have been introduced for interpretation of spectra that are based upon mathematical algorithms, which may assist quantitative analysis, such as factor analysis for determining the number of components in an array of mixtures or resolution-enhancement methods like derivative spectroscopy and peak deconvolution. Quantitative analysis is based upon Beer’s law. Several techniques have been developed like the calibration curve method, the standard addition method, the absorbance ratio method, the internal standard method, and the coincidence band method. For quantitative analysis in internal reflectance spectroscopy, it is essential that the sample is always in intimate contact with the same surface area of the crystal.
An experimental procedure [21] for the measurement of DC is described in the following paragraphs. After setting, cellulose strips were removed from the bracket-bonded adhesive flat surfaces, which would correspond under clinical conditions to the material in contact with enamel. These surfaces were analyzed using micro-multiple internal reflectance FTIR (micro-MIR FTIR) and the apparatus shown in Fig. 2.2. The flat surfaces were pressed against the sampling surface of a KRS 5 minicrystal of the micro-MIR FTIR accessory. The accessory cell (Fig. 2.3) was then placed on a FTIR spectrometer that was interfaced with a data station. Spectra from each resin surface were recorded under the following conditions: 4,000–400 cm−1 wave number range, 1–4 cm−1 resolution, and 20–50 scans. The mean micro-MIR sampling depth was estimated as 3 μm at 1,000 cm−1.



Fig. 2.2
Instrumentation for FTIR-ATR spectroscopy and FTIR microscopy

Fig. 2.3
FTIR accessory cell
The DC of each specimen was estimated on a relative percentage basis with the two-frequency method and the tangent baseline technique [21]. Aliphatic (linear) C=C bond-stretching vibrations at 1,638 cm−1 were chosen as the analytical frequency, whereas the aromatic (C..C) bond-stretching vibrations at 1,605 cm−1, which are not affected by the polymerization reaction, were selected as a reference frequency. In the cases where the adhesives contain no aromatic monomers, the C=O ester groups at 1,638 cm−1 or the N-H stretching vibration band at the region near 3,380 cm−1 was chosen as a reference frequency [21].
The % DC is obtained from the relationship that % DC = 100 (1 − RDB), where RDB is the fraction of residual C=C double bonds. The RDB can be estimated from an equation [21] containing four peak areas: (1) A p (C=C), the net peak absorbance area for the set material at 1,638 cm−1, (2) A m (C=C), the net peak absorbance area for the unset material at 1,638 cm−1, (3) A p (C..C), the net peak absorbance area of the set material at 1,605 cm−1 and (4) A m (C..C), the net peak absorbance area for the unset material at 1,605 cm−1.
Limitations
The IR method is essentially a surface analytical technique with a mean sampling depth of 3 μm, and consequently the middle zone of minimum conversion in the adhesive cannot be analyzed. Thus, the DC has a poor predictive value for the biological properties of orthodontic adhesives owing to the unique setting characteristics of these materials, noted in the following paragraph, which differentiate them from restorative resins.
2.2.3 Leaching
Background
Besides the lack of available evidence on biological properties of adhesives, achievement of a consensus on their behavior is complicated by the unique mode of application of these materials, which involves thin layers with distinctive setting characteristics [22]. These factors, which differ substantially between orthodontic adhesives and restorative composite resins, may modify the qualitative and quantitative aspects of leaching. Therefore, evidence derived from associated disciplines such as restorative dentistry, where there is an abundance of data on the DC of composite resins, may be irrelevant to the orthodontic analogue.
There has been only limited assessment [23] of the biological properties of the constituent components of composite resins on an individual basis. Inconclusive evidence has been found because various material constituents tested in an isolated form may produce different effects compared to the material as a whole [24]. This may be attributed to alteration of the reactive status of some components by the simultaneous presence of other constituents and the interactions of these substances during aging.
Lastly, the cell type utilized in assaying the biological effects of resins is crucial for the interpretation of results. In the past, immortalized mouse cell lines or even cancer cells have been used in relevant biocompatibility testing for biomaterials. Obviously any extrapolation of the findings of these studies to the normal human tissue is highly problematic.
Description
Monomer leaching has usually been investigated with high pressure liquid chromatography (HPLC), which is one of the most powerful tools in analytical chemistry, with the ability to separate, identify, and provide quantitative information about the compounds that are present in any sample that can be dissolved in a liquid. Today, trace concentrations of compounds, as low as parts per trillion (ppt), are easily obtained. HPLC can be applied to pharmaceuticals, food, nutraceuticals, cosmetics, environmental matrices, forensic samples, and industrial chemicals, in addition to dental polymers.
With liquid chromatography, a material for analysis (analyte) passes down a chromatographic column under the action of gravity, and separated colored bands are observed. The bands correspond to different chemical species that were originally contained in the test sample. Separation is based on chemical attraction of some species or compounds to the particles of a stationary phase, which causes these species to move more slowly in the column, while other species are attracted to the solvent (mobile phase) in the column and move more rapidly. Thus, chemical species in the analyte are distributed or partitioned between the mobile phase and stationary phase, creating the separation into bands.
Liquid chromatography can be performed by three primary approaches. In all cases, the analyte must be dissolved into a liquid that is then transported by the solvent onto or into the chromatographic device:
1.
The sample is “spotted” onto, and then flowed through, a thin layer of chromatographic particles fixed onto the surface of glass plates. The sample appears black, but is actually composed of yellow, red, and blue dyes. The bottom edge of the plate is placed in a solvent. The flow is created by solvent diffusion through the dry particle layer (capillary action) and movement up the glass plate. This is called thin layer chromatography (TLC).
2.
The sample is “spotted” onto paper to which solvent is added to create flow. This is called paper chromatography.
3.
In the most powerful approach, the sample passes through a column or device containing appropriate particles, which are called the chromatographic packing material, stationary phase, or adsorbent. Solvent flows continuously through the column. At a point in time, an injection of the sample solution is made into the solvent stream, which then carries the sample through the column. The compounds in the sample can then be separated because they travel at different individual speeds through the device. The designation of HPLC originally referred to the need for high pressure to generate the flow required for liquid chromatography in packed columns.
Specimen preparation is identical to that for DC specimens, with the exception of applying a cellulose strip to the bracket base and background surface so that recovery of the adhesive layer separately from the bracket is possible. Specimens are immersed in sterile tubes containing 50 mL of 0.9 % w/v saline solution and stored at 37°C for 2 months. During immersion, the solution is agitated twice daily. At the end of this period, 40 mL of eluent is removed from each solution, and the saline samples are processed for HPLC. The analysis is performed using methanol/water (4:1) mobile phase ratio, isocratic elution mode, 1 mL/min flow rate, and detection at 254 nm. The column is calibrated with known concentrations (standards) of TEGDMA and Bis-GMA solutions of each monomer in methanol. Linear fittings of the calibration curves are used to calculate the concentration of monomers in the saline solution from the area of chromatographic peaks at the corresponding retention time.
2.2.4 Fluoride Release
Background
It is well known that the presence of orthodontic appliances impairs the efficacy of oral hygiene, increasing the incidence of demineralization of tooth surfaces. Loss of the mineral surface of the tooth results in clinically detectable white spots, which are most pronounced at the gingival region where a greater accumulation of plaque occurs.
To overcome this problem, protective measures such as oral hygiene instruction, mechanical removal of plaque, and the topical application of fluoride agents have designed [25]. However, the efficacy of those measures depends on patient cooperation and compliance. For this reason manufacturers incorporate fluoride into orthodontic adhesives in order to achieve high levels of fluoride release.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


