Fig. 5.1
(a) Interaction of glass ionomer cement with MTA leading to failure of glass ionomer and (b) layering of MTA with zinc oxide eugenol with a resultant retardation of setting of MTA (Reprinted from Camilleri [9], copyright 2011, with permission from Elsevier)
5.2.1.2 Base Materials
Zinc oxide eugenol-based cements are used as temporary filling materials and come into contact with MTA when it is used for pulp capping. Zinc is a retarder of cement hydration [47], and zinc salts form calcium hydrozincate (Ca(Zn(OH)3H2O)2), an insoluble hydroxide in alkaline solution that creates a coating on MTA particles. In addition, zinc oxide retards the hydration of tricalcium silicate, although it does not interfere with the tricalcium aluminate/gypsum reaction. Zinc is incorporated into the calcium silicate hydrate gel phase [46], and the migration of zinc from zinc oxide cement into MTA has been reported. Furthermore, MTA in contact with intermediate restorative material (IRM) (Dentsply Caulk, Milford, DE, USA) exhibits a high degree of porosity resulting from incomplete hydration (Fig. 5.1b) [9].
5.2.1.3 Composite
The shear bond strengths of different composite adhesive systems to white MTA has been compared [6] with the conclusion that an etch-and-rinse adhesive system was preferred when placing compomer materials upon white MTA because it exhibited significantly higher shear bond strength values than self-etch adhesive systems. MTA used as pulp capping material necessitates the layering of MTA with composite for immediate restoration of the tooth. The use of bonding agent has been shown to reduce gaps at the material interface. Contact with both bonding agent and composite can reduce the micro-hardness of MTA in the early stages of its hydration [65].
5.2.2 Intra-canal Medicaments
When used inside the root canal for furcal repair and apexification procedures, MTA may come into contact with medicaments such as non-setting calcium hydroxide paste. No consensus has been reached on whether the calcium hydroxide in medicaments affects the sealing ability of MTA [20, 58]. However, calcium hydroxide does create an alkaline environment, which increases the porosity and un-hydrated microstructure of MTA [49], although no microstructural changes have been observed within MTA following contact with calcium hydroxide paste. On the other hand, migration of silicon and aluminium from MTA into calcium hydroxide medicaments has been observed [9].
5.2.3 Intra-canal Solutions
5.2.3.1 Bleaching Agents
MTA has been reported to be more easily displaced when in contact with sodium perborate mixed with saline, Superoxol (Sultan Healthcare, Hackensack, New Jersey, USA), and sodium perborate mixed with Superoxol, whereas IRM was not affected by the treatments used clinically for tooth bleaching [34]. Bleaching agents have also been shown to affect the elemental distribution of MTA [64]. A decrease in calcium and an increase in silicon were observed, and this tendency was especially pronounced when higher concentrations of hydrogen peroxide were used. The acidic conditions induced by bleaching agents also deteriorate the surface of MTA. Overall, these findings suggest that MTA is not an effective barrier during tooth bleaching and should be protected by a more suitable material.
5.2.3.2 Chelators
Calcium chelators used as irrigating solutions affect the strength and microstructure of MTA. It has been reported that ethylenediaminetetraacetic acid (EDTA) significantly reduced the hardness and flexural strength of MTA compared with distilled water. Indeed, when in contact with EDTA, set MTA had a reduced Ca/Si molar ratio, a reduced calcium hydroxide production, lack of biocompatibility and reduced micro-hardness [31]. Therefore, after use of EDTA in the root canal system, a final flush with distilled water is advocated before placement of MTA [1]. EDTA and BioPure MTAD (Dentsply, Tulsa Dental Specialities, Tulsa, Oklahoma, USA) decreased the sealing efficacy of MTA 24 h after placement to suggest it may be beneficial to seal furcal repair sites where MTA has been used with a protective lining material before commencement of chemo-mechanical root canal preparation [66].
5.2.3.3 BioPure MTAD
BioPure MTAD-treated MTA surfaces exhibit greater surface roughness and more calcium loss when compared with EDTA treatment. Decomposition of the particle-binding hydration phases by acid erosion raises potential concern over the strength and sealing properties of MTA-repaired perforations following final irrigation using BioPure MTAD [57]. The seal of MTA used to repair root perforations has been reported to be negatively affected by irrigating solutions. Fluid conductance was affected by the type of irrigating regime used with mixtures of sodium hypochlorite and EDTA/MTAD resulting in increased leakage using the fluid filtration method to assess sealing ability of MTA [66].
5.2.3.4 Sodium Hypochlorite
NaOCl is used routinely as an irrigant in root canal treatment. Interestingly, its interaction with MTA has not been investigated extensively. However, NaOCl has been shown to enhance the push-out bond strength of MTA in the early stages of hydration [26]. MTA exhibits dark brown discoloration in contact with sodium hypochlorite solution [10], (Fig. 5.2). It is postulated that since sodium hypochlorite is reduced to sodium chloride, the oxygen present can destabilise the bismuth oxide in MTA rendering it reactive with the atmospheric carbon dioxide to be converted to bismuth carbonate, which is light sensitive and thus turns black when exposed to light. The sensitivity of MTA to different light sources has been demonstrated [67, 68].


Fig. 5.2
Photograph of dried bismuth oxide powder after immersion for 24 h in sodium hypochlorite solution, showing surface crystalline deposits
5.2.4 Root Canal Sealers
MTA in contact with formaldehyde exhibits black discolouration Marciano MA, Hungaro Duarte MA University of Bauru (Personal Communication). Formaldehyde is released from resin-based sealers containing hexamethylenetetramine, such as AH 26, and Sealer 26 as a result of a chemical reaction between bisphenol A resin and hexamethylenetetramine.
5.2.5 Acid Etching
It is well known that exposure of MTA to a low pH environment will influence its physical and chemical properties [14, 70]. It is therefore not surprising that in the laboratory environment application of acid etch has adverse effects in the short term, on the push-out bond strength [56], porosity, micro-hardness [37] and compressive strength [28] of MTA, suggesting it would be better to postpone restorative procedures to allow more advanced hydration of the cement. Over longer time periods after placement, the application of etchants on some brands of MTA does not appear to affect its compressive strength [29]; however, other brands were more susceptible to etchant and have a reduced strength.
Following the laboratory evaluation of acid etchant on various materials [44], it was concluded that etching of MTA did not improve its shear bond strength to composite resin and that the surface etching of MTA was not necessary prior to composite placement using a total-etch adhesive resin. Furthermore, it is advised that when MTA is used in vital pulp therapies, it is better to cover the material with glass ionomer cement. However, it must be emphasised that these reports are based on laboratory investigations and that clinical trials have not been reported on the use and effects of acid etchant on MTA in a clinical setting.
The effects of exposing MTA to acid etching have been demonstrated by SEM analysis. In general, a selective loss of matrix from around the crystalline structures of MTA has been observed resulting in a relatively uniform ‘honeycomb’ pattern without penetrating deeply or removing substantial amounts of cement. In addition, etching has revealed crystalline structures such as plate-shaped and laminated crystals on the MTA surface; however, needlelike crystals have been reported to be missing (Fig. 5.3a, b) [28]. Further investigation on the material microstructure after exposure to acid etch in order to identify crystal morphology is necessary. The significance of these morphological changes is unclear.
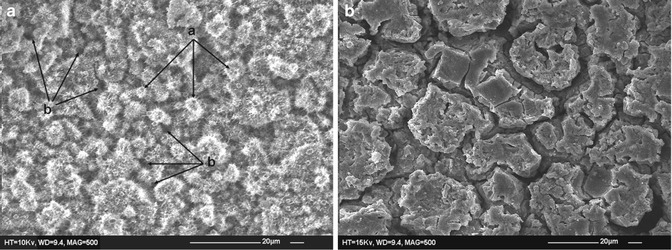

Fig. 5.3
(a) Intact surface of unetched mineral trioxide aggregate after 24 h. Irregular needlelike crystals that cover globular formulations (a) and cross sections of several micro-channels (b) can be seen (mag. 500×). (b) Etched enamel surface after 24 h. Selective loss of matrix from around the crystalline structures and relatively uniform honeycomb etched pattern with minimal loss of the cement can be seen. No needlelike crystals were observed (mag. 500×) (Reprinted with permission from Kayahan et al. [28]. © 2009 International Endodontic Journal)
5.2.6 Blood
The contamination of MTA by blood has been investigated in a number of laboratory studies in terms of the effect on its physical properties, leakage, displacement, marginal adaptation and colour. There is little doubt that blood contamination on the surface of MTA and, in particular, when incorporated into the material is detrimental to its hydration and thus its ultimate physical properties and performance. Unfortunately, MTA is often placed in contact with vital tissues that ooze blood/serum (pulp capping) or in situations where blood pools on its surface (root-end filling), and the impact of blood contamination is important and must be minimised.
5.2.6.1 Physical Properties
Mixing MTA with blood has a negative effect on its surface hardness, microstructure [41] and compressive strength [42]. Essentially, it has been reported that when blood becomes incorporated into MTA, its compressive strength is reduced with the result that in clinical situations in which blood becomes mixed with MTA, its physical properties are likely to be compromised [42], even when used with accelerators [43]. Similar effects have been reported when foetal bovine serum was used to contaminate the surface of MTA [30].
The hydration state of MTA mixed with blood has also been reported [39] with specimens partially mixed with blood being more completely hydrated than those mixed entirely with blood and less than specimens hydrated completely with water. Lack of formation of the crystalline calcium hydroxide in the early stage of the hydration process and the absent of acicular crystals, characteristic of ettringite crystals, in blood-contaminated specimens are common findings (Fig. 5.4).
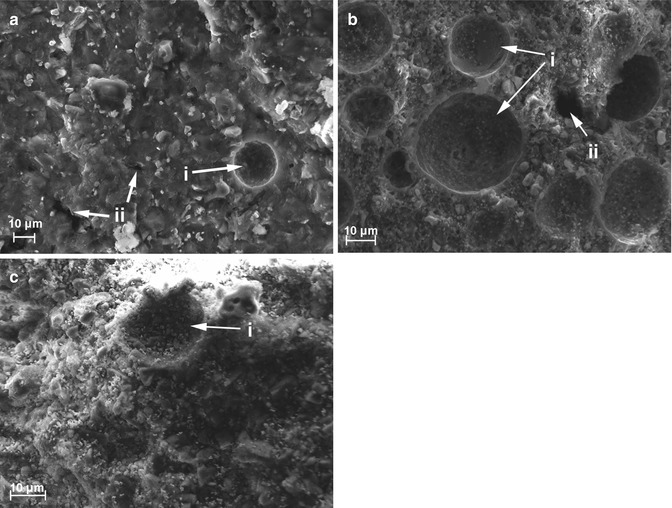

Fig. 5.4
Scanning electron microscopy images of mineral trioxide aggregate specimens mixed entirely with water (a), partially (b) and entirely (c) with whole, fresh human blood. Porosity (i) and presence of cross sections of micro-channels (ii) can be observed (Reprinted with permission from Nekoofar et al. [39]. © 2011 International Endodontic Journal)
5.2.6.2 Leakage
In laboratory studies, it has been reported that blood contamination of the surface of MTA increases leakage of root-end fillings when using tracer dyes [25]. An earlier study did not exhibit any leakage when root-end restorations were contaminated with blood and leakage was assessed using dyes [63].
5.2.6.3 Displacement
5.2.6.4 Marginal Adaptation
It has been reported that exposure to blood during setting has a negative effect on marginal adaptation of MTA [52].
5.2.6.5 Colour
Contamination of MTA with blood has been shown to have an adverse effect on tooth colour [33].
In summary, it is likely that the detrimental effects of blood on MTA will have a negative impact on its physical characteristics and thus on its performance in a variety of laboratory tests. From a clinical perspective it seems sensible to avoid blood contamination if possible.
5.2.7 Bone Grafting Materials
Demineralised and mineralised graft materials appear to have a differential effect on the micro-hardness of white MTA. White MTA micro-hardness values when in contact with Bio-Oss (Geistlich Pharma, Princeton, New Jersey, USA), MinerOss (BioHorizons, Markham, Ontario, Canada) and Puros (Zimmer Dental, Carlsbad, California, USA) have been reported to be lower than those for OraGraft (Salvin Dental Specialities, Charlotte, North Carolina, USA) and control groups regardless of incubation period [53].
5.2.8 Variable pH
A number of laboratory studies have evaluated the physical properties of MTA specimens following exposure to a range of acidic environments during hydration on the basis that in some situations the tissues or fluids that come into contact with MTA are acidic. The mean pH of pus from periapical abscesses was generally acidic, although some samples were neutral and some were alkaline [40]. It has been reported that surface hardness of MTA was reduced in an acidic environment [37] as is push-out strength [56]. Leakage of root-end fillings has also been reported to be affected by low pH [50]. The effect of acidic environment on the dislodgement resistance of MTA when used as a perforation repair material has been compared, and it was concluded that its dislodgment resistance was significantly reduced after exposure to acid [23]. Alkaline pH has also been reported to have a negative impact on push-out strength [51], surface hardness and porosity [49].
5.2.9 Saliva
Since MTA is known to have relatively poor physical properties in terms of strength and wear resistance, it has never been recommended for use when in contact with saliva. Thus, the contamination of MTA with saliva is unlikely in most situations, but may occur if the material is used to repair perforations that communicate with the mouth, i.e. in perforations occurring within periodontal pockets. Despite this, a number of laboratory studies have been conducted on salivary contamination of MTA with conflicting results. It has been reported that saliva reduces [24], increases [36] or has no effect [25] on leakage of MTA when used as a root-end filling material. The leakage of MTA when exposed to saliva and used as an orifice barrier [71] and an orthograde filling material [3] has also been evaluated with conflicting results. Overall, it must be remembered that the validity of leakage studies has been questioned and the conclusions of these and other similar studies are likely to be meaningless.
5.2.10 Tissue Fluid and Simulated Tissue Fluid
Mineral trioxide aggregate used as a root-end filling comes into contact with tissue fluid before complete hydration is achieved. When MTA is in contact with tissue fluid, the setting time is extended and in certain cases the material may not set at all [11, 19]. Immersion in tissue fluid has been reported to result in incomplete setting of MTA as the presence of phosphates in solution retards its hydration. The retardation is induced by the formation of insoluble hydroxides in the alkaline solution. The insoluble hydroxides form a coating over the cement particles. The adsorption of phosphate ions on the surface of the clinker phase or on the hydration product is thought to result in the precipitation of calcium phosphates [46]. Glucose, which is present in Hank’s balanced salt solution, is also a known retarder of cement hydration [46].
The retardation of setting is apparent under the scanning electron microscope when the surface microstructure of MTA in contact with Hank’s balanced salt solution was investigated. MTA in contact with simulated body fluid exhibits no evidence of hydration (Fig. 5.5). The cement in contact with simulated body fluid exhibits micro-cracking, which is caused by expansion of the cement [11].
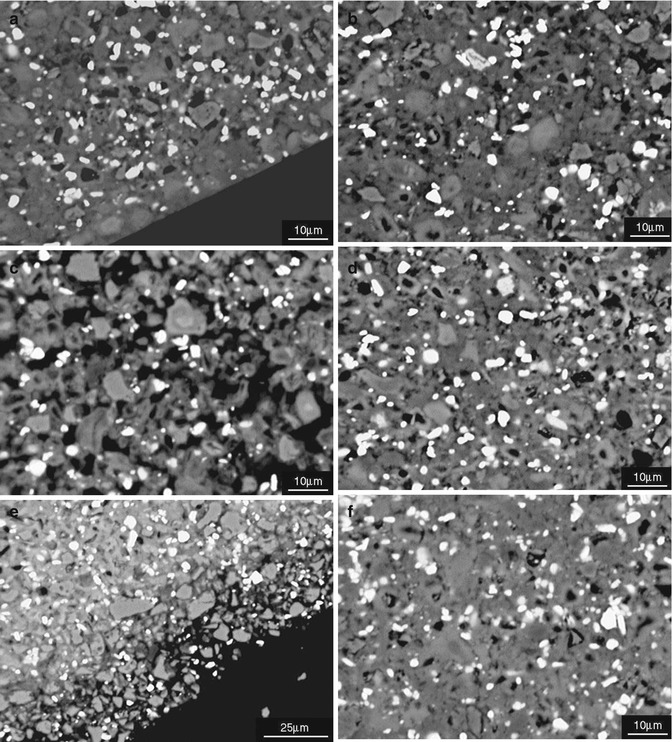

Fig. 5.5
Scanning electron micrographs of MTA stored in different environmental conditions (a, b) dry, (c, d) immersed in water (e, f) immersed in HBSS. (a, c, e) Depict the outer region whilst (b, d, f) the core region (Reprinted from Camilleri et al. [11]. © 2013 International Endodontic Journal. Published by John Wiley & Sons Ltd)
When set MTA is stored in Hank’s balanced salt solution, the pH of the storage solution becomes less alkaline. This could be due to the presence of buffers in simulated body fluids. These buffers are added to these solutions to maintain their pH. Regardless of the less alkaline pH, calcium ion release has been demonstrated in Hank’s balanced salt solution. The leaching of calcium is higher in simulated body fluid than in distilled water both when tested after 1 day of immersion and at 28 days of material contact with the solution [8, 17].
The compressive strength of MTA stored in Hank’s balanced salt solution is lower than when stored in a humid environment or immersed in water [18]. The dimensional stability of MTA in contact with different soaking solutions has been investigated and a net expansion has been reported when the materials were placed in contact with physiological solutions [8, 59]. MTA materials achieved approximately half of their final linear setting expansion by 300 min, with approximately 75 % of expansion occurring by 460 min and the final 25 % of total expansion occurring between 460 min and 24 h [59].
5.2.11 Effect on Dentine and Pulp
MTA releases calcium hydroxide as a by-product of hydration. The calcium hydroxide has a beneficial effect on the pulp, and dentine bridge formation has been reported. Random controlled clinical trials have shown that MTA is associated with the best clinical outcomes when used as a pulp capping material and for apexification/apexogenesis procedures (as discussed in Chap. 3; Table 3.1). The metallic ions released by set and setting MTA when placed clinically, may release dentine matrix components that potentially influence cellular events for dentine repair and regeneration [61].
When in contact with dentine, MTA altered the toughness more than the strength and stiffness of dentine after ageing in 100 % relative humidity. Dentine toughness is attributed to its collagen matrix; thus, MTA seems to affect the dentine collagen matrix [54]. Prolonged contact of mineralised dentine with MTA has an adverse effect on the integrity of the dentine collagen matrix. However, the amount of collagen extracted was limited to the contact surface. Clinicians are thus advised to use MTA with caution when it is applied to thin dentinal walls [32].
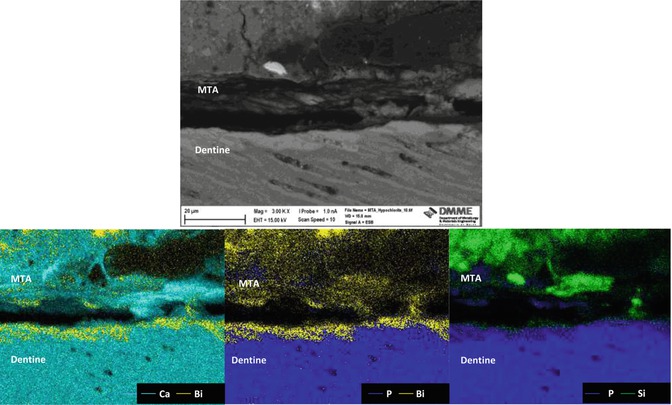
Along the material-dentine interface, MTA forms a taglike structure that is composed of either Ca- and P-rich crystalline deposits or the material itself. The width of a Ca- and Si-rich layer detected along the dentine layer of the material-dentine interface increases over time [21]. The selective diffusion of silicon, calcium and phosphorous across the cement-dentine interface has been demonstrated [16]. The movement of calcium is difficult to show using scanning electron microscopy and elemental mapping since calcium is present in both the material and dentine. However, elemental migration is clearly shown across the interface (Fig. 5.6). Together with calcium, silicon and phosphorus, migration of bismuth is also evident. Migration of bismuth into dentine can be problematic as bismuth oxide has been shown to react with collagen resulting in a change in colour of both MTA and the tooth [35] (Fig. 5.7).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses