Bisphosphonate (Trade name, manufacturer)
Common indications for use
Dosage
Relative potencya
Etidronate (Didronel, P&G)
Paget’s disease
300–750 mg/die
× 6 months
1
Alendronate (Fosamax, Merck)
Osteoporosis
10 mg/day
70 mg/week
1,000
Risedronate (Actonel, P&G)
Osteoporosis
5 mg/day
35 mg/week
5,000
Ibandronate (Bonviva, Roche)
Osteoporosis
2.5 mg/day
150 mg/month
10,000
Pamidronate (Aredia, Novartis)
Bone metastases, Multiple myeloma
90 mg/3 weeks
100
Zoledronic acid (Zometa, Novartis)
Bone metastases, Multiple myeloma
4 mg/3 weeks
100.000
Zoledronic acid (Aclasta, Novartis)
Osteoporosis
5 mg/year
100.000
Until a few years ago, bisphosphonates were generally considered to be well tolerated, with minimal adverse effects. Prior to 2003, they were far removed from the concerns of dentists and oral and maxillofacial surgeons; however, in that year a side effect of NBP treatment, later referred to as bisphosphonates-induced osteonecrosis of the jaw (BIONJ), was documented and has since frequently been reported [10–14]. BIONJ is defined as the presence of exposed bone in the oral cavity that does not regress within 8 weeks in patients who are currently being treated or who were previously treated with systemic intravenous or oral NBPs and who have not undergone radiation therapy to the maxillofacial region. BIONJ can seriously affect the quality of life by producing significant degrees of morbidity [15]. Estimates of the cumulative incidence of BIONJ in cancer patients receiving i.v. BP range from 0.8 to 18.6% [15, 16]. The frequency of BIONJ in osteoporotic patients is between 0.01 and 0.04%. In the group of patients on NBPs who have undergone dental extractions, the frequency in the range of 0.09–0.34% [13, 14]. It is therefore of critical importance that dentists be aware of their role in the early diagnosis of osteonecrosis, its prevention, and the treatment options. In addition, a profound knowledge of the clinical recommendations is needed in order to advise patients on NBPs regarding appropriate dental treatment.
The role of the dentist can be summarized as follows:
1.
Be aware of the risk of dento-alveolar surgical procedures in the development of BIONJ.
2.
Recognize the clinical and radiographic features of osteonecrosis.
3.
Identify the local and systemic risk factors in patients on NBPs that place them into the low- or high-risk group for BIONJ.
4.
Adopt preventive strategies in patients on NBPs, and especially in those who require dento-alveolar surgical procedures.
5.
Consider the predictive value of biochemical markers of metabolic bone activity in determining the risk of BIONJ.
10.2 Diagnostic Considerations
Since the publication of the first case series, it has been clear that the location and function of the maxillary bones explained their vulnerability to BIONJ. In fact, the majority of BIONJ cases involve patients who have undergone invasive dental procedures such as dento-alveolar, periapical, periodontal, or implant surgery. BIONJ exclusive effects on the mandible and maxilla are due to the fact that bisphosphonates become highly concentrated in these metabolically active bones [17]. The bones of the jaw have a higher blood supply than other bones in the body and a higher rate of bone turnover, and thus higher osteoclast activity. In addition, chronic dental disease, invasive dento-alveolar treatments, and the thin mucosal barrier protecting the bones of the jaw explain why BIONJ is manifested exclusively in the oral cavity [18].
In daily practice, it is well known that patients frequently do not inform the dentist of the drugs they are currently taking or medications that they took in the past which have since been discontinued. Thus, before a patient undergoes oral surgery, it is essential that the dentist ask the patient whether he/she suffers from any pathological bone syndromes that are or might be treated with NBPs. These, obviously, include metabolic bone disease (osteoporosis, osteopenia, Paget’s disease), bone metastasis of solid tumors, and multiple myeloma. In addition to the NBP formulation, the dosage, frequency, and duration must be registered in the patient’s medical record, as they are critical factors that may contribute to the development of osteonecrosis. Moreover, and particularly in the case of elderly or cancer patients, a detailed pharmacological history is essential and may require that the dentist communicate with the patient’s health care practitioners (physician, family practitioner, gynecologist, endocrinologist, oncologist) who may be prescribing NBPs [19].
10.3 Clinical and Radiographic Features of BIONJ
It is mandatory for the dentist to recognize the signs and symptoms of BIONJ as an emergent maxillary disease. A clinical staging scheme, proposed by the American Association of Oral and Maxillofacial Surgeons (AAOMS), serves as the current clinical guidelines for the diagnosis of BIONJ (Table 10.2) [15]. These guidelines consist of five stages. Stage 1 is the “patient at risk”, which refers to asymptomatic patients, i.e., without bone exposure, who have been treated with NBPs. Stages 0–3 describe the features of necrotic bone exposure, from early to advanced, and the association with pain, infection, fistula, and fracture. In patients with stage 0 disease, there is discomfort and non-specific oral symptoms without apparent exposure of necrotic bone. The first clinically evident sign of BIONJ is single or multifocal exposed necrotic bone in the jaws. The process is more common in the mandible, but, alone or in combination, may involve the maxilla too (Fig. 10.1).
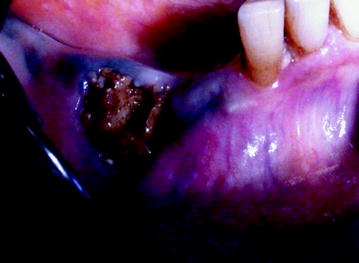
Table 10.2
Clinical staging scheme for the diagnosis of BIONJ according to the AAOMS (2008)
|
Staging
|
Clinical features
|
|---|---|
|
Stage 0
|
Regional or diffuse osteosclerosis in clinically symptomatic areas, dense confluence of cortical and cancellous bone, prominence of the inferior alveolar nerve canal, markedly thickened and sclerotic lamina dura
|
|
Stage 1
|
Cortical disruption, lack of bone fill after extraction and non-healing alveolar socket
|
|
Stage 2
|
Enlarged osteolytic areas in symptomatic patients
|
|
Stage 3
|
Bone sequestrum, pathologic fracture, oral-antral/oral-nasal communication, osteolysis extending to the inferior border of the mandible or sinus floor
|

Fig. 10.1
Stage I BIONJ consequent to molar extraction
Although most cases of exposed necrotic bone are the consequence of unsuccessful healing of alveolar bone after tooth extraction, patients wearing a denture or with mandibular or palatal tori covered with a thin mucosa are also at risk. In such cases, a non-healing mucosal breach, e.g., due to an unstable denture or small traumatic injury, could be a portal for oral bacteria to access the bones of the jaw (Figs. 10.2, 10.3). While the exposed necrotic bone is not painful in itself, the onset of a secondary infection will cause pain and may lead to cellulitis and abscess formation (Fig. 10.4). The differential diagnosis includes inflammatory, reactive, cystic and neoplastic pathologies that can involve the jaws.
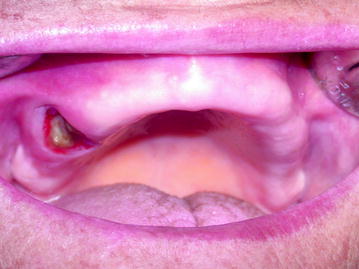
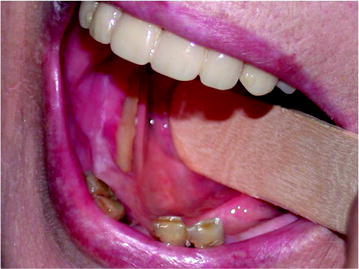
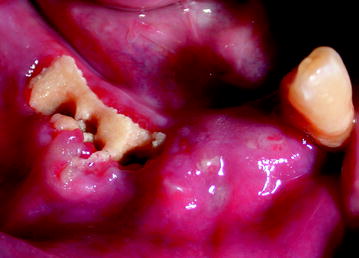

Fig. 10.2
Stage I: Maxillary exposure in a denture wearing patient

Fig. 10.3
Mandibular exposure associated with thin mucosal coverage

Fig. 10.4
Stage II: BIONJ consequent to teeth extraction
Pathological fractures can occur in BIONJ and may go unrecognized as such if debridement surgeries have reduced the structural integrity of the mandible (Fig. 10.5). Other symptoms of BIONJ are oral-cutaneous, oral-antral, and oral-nasal fistulas, which appear in the advanced stages (Fig. 10.6).
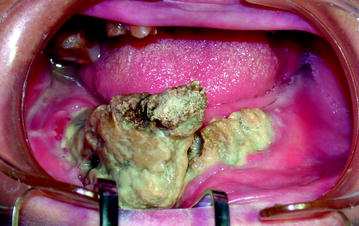
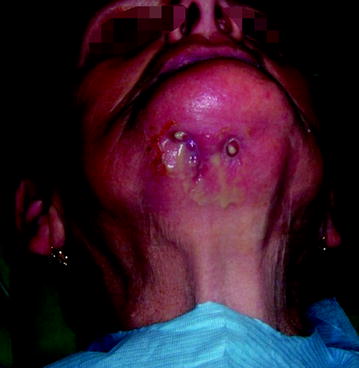

Fig. 10.5
Stage III: Pathological mandibular fracture of the symphysis

Fig. 10.6
Stage III: Oral cutaneous fistula in a patient with stage III disease
The radiologic features of the early stages of BIONJ illustrate the increasing bone mineral density that follows inhibition of osteoclast activity and proliferation (Fig. 10.7). In the exposed bone, the progressing osteonecrosis together with secondary infection results in focal areas of demineralization that may lead to the formation of a sequestrum (Figs. 10.8, 10.9).
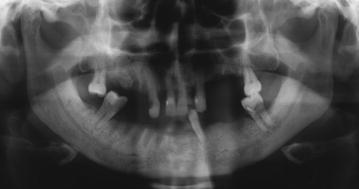
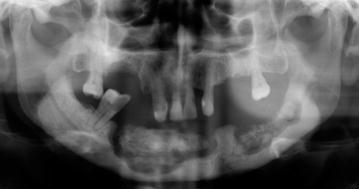
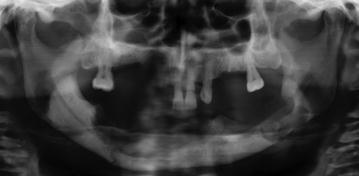

Fig. 10.7
Non-healing alveolar socket in patient with necrotic exposed bone

Fig. 10.8
Mandibular bone sequestra

Fig. 10.9
Mandibular pathologic fracture
10.4 Risk Factors in the Development of BIONJ
A risk assessment of BIONJ is strongly recommended in patients taking NBPs who require invasive dental procedures [20]. High-risk patients are those receiving high-dose and high-potency NBPs as chemotherapeutic agents. In this group, the route of administration may consist of monthly treatment with high intravenous doses of pamidronate or zoledronic acid, or oral bisphosphonates, e.g., ibandronate, (Bondronat, Roche) 50 mg/day. Patients in the low-risk group are those on bisphosphonates for the treatment of osteoporosis, osteopenia, and Paget’s disease. These patients typically receive 5 mg zoledronate (Aclasta Novartis)/year or 3 mg ibandronate (Bonviva, Roche)/3 months.
In both groups of patients, the inability of soft tissues to envelop the extraction socket—as may occur in patients undergoing extraction procedures without any preventive measures—results in exposure of the underlying bone and consequent infection. The final effect is a progressive necrotizing, unremitting osteomyelitis. Oral NBPs for osteoporosis treatment produce milder BIONJ than intravenous forms of these drugs since the degree of necrosis is related to the potency and dosage of NBPs. For metastatic cancer, the dose is 4–12 times higher than for osteoporosis [21]. Another factor that must be considered are differences in the mean period of BIONJ onset in patients on NBPs: typically, 9 months for zoledronate and 30–52 months for alendronate [22].
Moreover a relevant number of secondary local and general risk factors have been identified. The addition of secondary factors can potentially shift a patient from a low to a high risk of developing of BIONJ. These local risk factors include:
The role of periodontal and periapical diseases is not clear. Hoff et al. reported that cancer patients with a history of periodontal and dental abscesses have a seven-fold higher risk of BIONJ [25]. This was refuted in other studies, especially when oral surgery was avoided in these patients [24, 26].
The general risk factors involved in BIONJ are:
-
Demographics (age > 60 years): Since blood circulation and the ability to recover from trauma are decreased in older patients, advanced age is considered to place patients at increased risk.
-
Female gender. Women are at higher risk because they are more likely to receive NBPs, i.e., for the treatment of osteoporosis [27].
-
Other drugs. Concomitant therapy with corticosteroids, chemotherapeutics, or thalidomide increases the risk of BIONJ because the molecular mechanisms of these medications include inflammatory and immune suppression involving osteoclasts, osteoblasts, and osteocytes, thus increasing the risk of osteonecrosis, although this issue is debated in the literature [28].
-
Concomitant disease: Immunocompromise and uncontrolled diabetes are systemic conditions that can affect bone turnover.
-
Life-style related: Heavy tobacco consumption and poor oral hygiene are associated with the delayed healing of surgical wounds and extraction sockets [29].
-
Genetic factors: Single-nucleotide polymorphisms in the CYP2C8 gene influence the development of BIONJ in patients with multiple myeloma treated with NBPs [30].
Some of the above-mentioned risk factors can be eliminated or altered in patients on NBPs. Thus, for example, it is fundamental to encourage patients to stop smoking and to improve oral hygiene. If the patient’s systemic condition permits the discontinuation of corticosteroid treatment or the completion of chemotherapy, then the dental invasive procedure can be delayed to reduce the risk of BIONJ.
10.5 Preventive Measures in Oncologic Patients
Given the ineffectiveness of currently available BIONJ treatments, prevention of the precipitating dental risk factors, especially in cancer patients, seems to be the most rational approach [31, 32]. As noted above, based on the oncologist’s assessment, if the patient’s clinical condition allows the postponement of NBP therapy, the dentist then assumes a decisive role. He or she has to carry out a complete dental screening to identify the presence of: odontogenic infections, compromised teeth that require intervention, periodontal disease, unstable dentures, and in limited cases, extraction. All patients need instructions on adequate hygiene control. At this point, all endodontic, conservative, and periodontal procedures should be performed. During and after medical treatment with intravenous NBPs, patients should not undergo any dental procedure without first consulting the treating physician [17, 33]. The administration of bisphosphonates by the oncologist can begin 6–8 weeks after the invasive procedure, when complete soft-tissue healing has been documented.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


