http://evolve.elsevier.com/Haveles/pharmacology
Dental treatment of the pregnant or nursing woman is always of special concern to dental hygienists. Pregnant women often need additional dental treatment during their pregnancies, and in addition, that treatment must be carefully planned. Many questions about drug therapy for the pregnant or breastfeeding woman arise. The literature, unfortunately, does not provide all the answers. This chapter attempts to offer guidelines for determining the relative risk when drugs are prescribed for the pregnant woman or nursing mother. No unnecessary drug should be administered to the pregnant woman. If a drug is to be administered, the risk to the fetus must be weighed against the benefit to the woman. An adequate health history, including whether a woman might be pregnant (puberty to menopause), should be taken at each dental appointment. Close coordination with the patient’s obstetric health care professional is recommended when questions about her potential use of drugs arise. Consultations should be documented in the patient’s chart. Box 25-1 lists the dental implications involved in managing a pregnant dental patient.
General principles
Two Main Concerns
Two main concerns must be addressed when considering whether to give a drug to a pregnant woman. The first is that the drug may be teratogenic. The term teratogen is derived from the Greek prefix terato-, meaning “monster,” and the suffix -gen, meaning “producing.” These two combine to give rise to the meaning of teratogen: “producing a monster.” The second is that the drug can affect the near-term fetus, causing the newborn infant to have an adverse reaction, such as respiratory depression or jaundice. A relatively new concern is the long-term (physiologic and psychological) consequences of in utero drug exposure that are not evident at birth.
History
In 1941, a relationship between getting German measles during pregnancy and blindness, deafness, and death of the offspring was noted. Scientists recognized that exogenous agents could affect the unborn fetus, producing congenital abnormalities. In 1961, a “harmless” sedative, thalidomide, available over the counter (OTC) in Europe, was taken by pregnant women. An increase in the rare birth defect phocomelia (shortness or absence of limbs) occurred shortly thereafter. Thalidomide was later implicated in these birth defects. Environmental factors are also thought to contribute to birth defects.
Pregnancy
Pregnancy Trimesters
Pregnancy involves three trimesters, each 3 months long. During the first trimester, the organs in the fetus are forming. This is considered the most critical time for teratogenicity. If abnormalities occur very early in development, spontaneous abortion is the usual outcome. With later exposure, abnormalities occur in the fetus. Often, a woman is unaware that she is pregnant for at least half of this trimester. Dental prophylaxis with detailed instructions and a visual examination of the oral cavity without X-rays should be performed if the patient is pregnant. Because the woman in the first trimester of pregnancy may feel nauseated at any time during the day or night (often referred to as morning sickness), other elective dental treatment should be avoided at this time.
The second trimester is an excellent time for the patient to receive both oral health instructions and another dental prophylaxis treatment, if needed. The patient’s periodontal status should be carefully evaluated. The patient is most comfortable during this trimester.
The third trimester is closest to delivery. The woman is beginning to feel uncomfortable, and it is difficult for her to lie prone for any length of time. If dental treatment is needed, she may feel more comfortable sitting or with the right hip elevated. In addition, this is the time when premature labor is most likely to begin. Drugs that may affect the newborn child should not be given during this trimester.
Teratogenicity
It is difficult to prove that a drug is teratogenic in humans (Box 25-2).
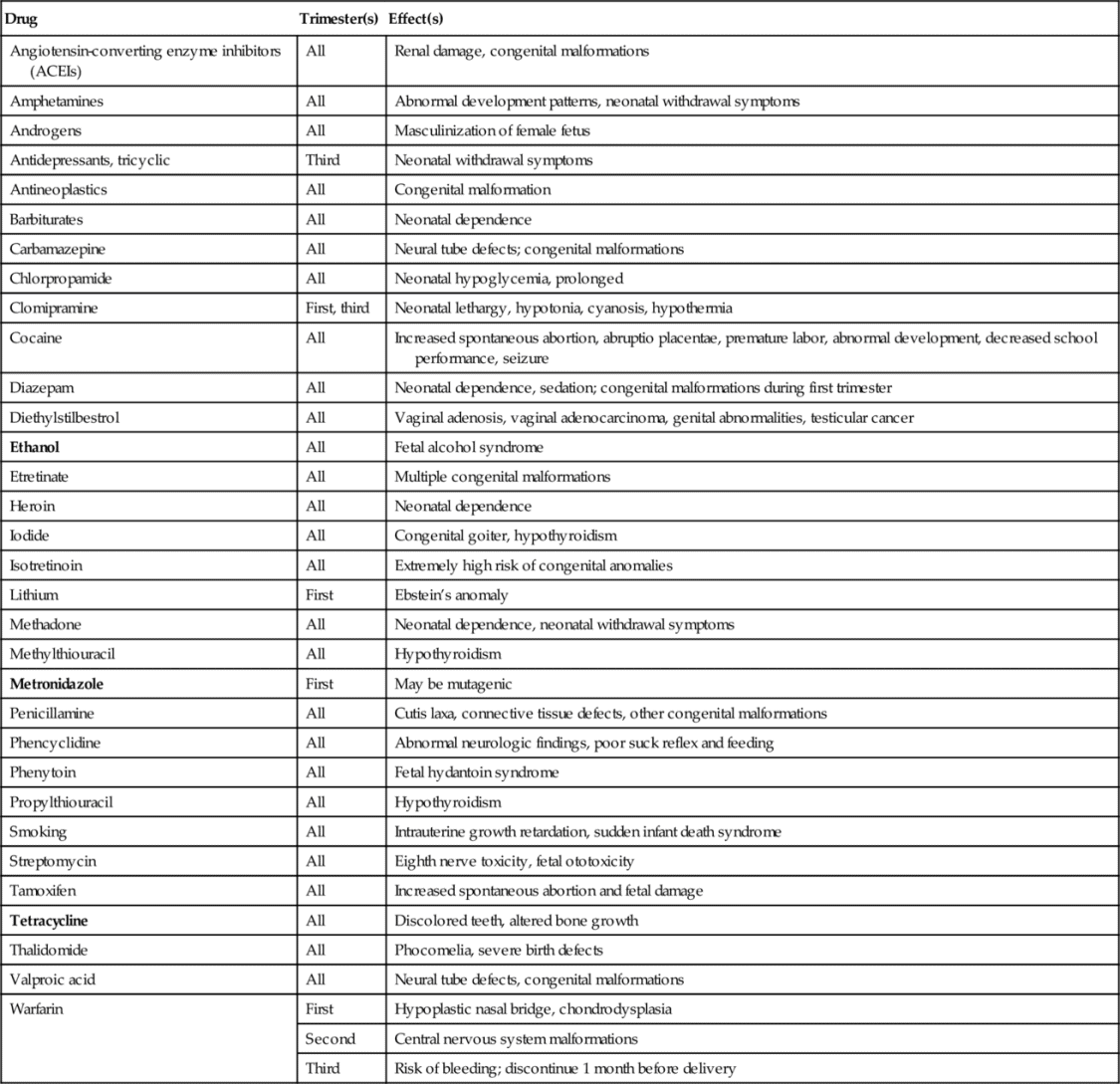
Drugs that are known teratogens include thalidomide, certain vitamin A analogs (isotretinoin), antineoplastic agents (busulfan, cyclophosphamide), oral anticoagulants (warfarin), lithium, methimazole, penicillamine, some antiepileptic agents (phenytoin, trimethadione, valproic acid), the tetracyclines, certain steroids (diethylstilbestrol, androgens), and ethyl alcohol. Table 25-1 lists selected drugs with adverse effects on the fetus.
U.S. Food and Drug Administration Pregnancy Categories
The U.S. Food and Drug Administration (FDA) has developed pregnancy categories A, B, C, D, and X for drugs. Each drug that is the subject of FDA regulation for pregnancy labeling is given a category based on its known potential for risk. Table 25-2 gives a summary of the criteria for the different categories. One should note that the availability of animal or human studies is a criterion. Category A is the safest, and category X should not be used in pregnant women. Categories B, C, and D fall between these two extremes.
Table 25-2
U.S. Food and Drug Administration (FDA) Pregnancy Categories For Drugs*
| Category | Description | Examples |
| A | Studies in pregnant women have failed to demonstrate a risk to fetus in first trimester and there is no evidence of risk in later trimesters; possibility of fetal harm appears remote. | Thyroid supplements (levothyroxine), vitamins (folic acid, riboflavin; vitamins A, D, and C†), potassium chloride |
| B | Animal studies have failed to demonstrate a risk to the fetus, and there are no adequate studies in pregnant women; OR animal studies show an adverse effect on the fetus but well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus. | Acetaminophen, acyclovir, opioids,‡ penicillins, cephalosporins, erythromycin,§ caffeine, cimetidine, insulin |
| C | Animal studies have shown an adverse effect on the fetus and there are no adequate studies in humans, OR no studies are available in either animals or women. Potential benefits may warrant its use. | Epinephrine, phenylpropanolamine, trimethobenzamide, aspirin,¶ atropine, promethazine, theophylline, lisinopril, disulfiram, propranolol, fluoxetine, amitriptyline, sulfonamides,¶ prednisone nonsteroidal antiinflammatory drugs (NSAIDs) |
| D |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses