http://evolve.elsevier.com/Haveles/pharmacology
No drugs are used more often in the dental office than the local anesthetic agents. Because their use can become routine, it is easy to forget that these agents have a potential for systemic effects in addition to the desired local effects. Dentists and, in most states, dental hygienists are responsible for the administration of local anesthetic agents when necessary. With this duty comes the need for an in-depth knowledge of the local anesthetic agents.
History
“Painless” dentistry, through the use of a local anesthetic, is a relatively recent development. It began with the observation that the indigenous people of the South American Andes chewed certain leaves that made them feel better. The active ingredient of the leaves was cocaine, isolated by Niemann in 1860. He noted that tasting this substance produced not only the loss of taste but also of the sensation of pain (Figures 9-1 and 9-2). In 1884, Koller noted that cocaine instilled in the eye produced complete anesthesia. Cocaine was immediately adopted for use in eye surgery. During this time, Sigmund Freud was also experimenting with cocaine and its effects on the central nervous system (CNS). CNS stimulation, toxicity, and the potential for abuse were quickly recognized as major problems with the widespread use of cocaine as a local anesthetic.
The search for a more acceptable local anesthetic for dentistry continued. Einhorn synthesized procaine in 1905, but it was not until many years later that its use in dentistry became common. In 1952, the amide lidocaine (Xylocaine) was approved by the Food and Drug Administration (FDA), and mepivacaine (Carbocaine) was approved in 1960. More recently, bupivacaine (Marcaine) has been made available for dental use. The search for the perfect local anesthetic agent continues.
Ideal local anesthetic
Although local anesthesia can be produced by several different agents, many are not clinically acceptable. The ideal local anesthetic should possess certain properties (Box 9-1). No local anesthetic agent in use today meets all of these requirements, although many acceptable agents are available.
Chemistry
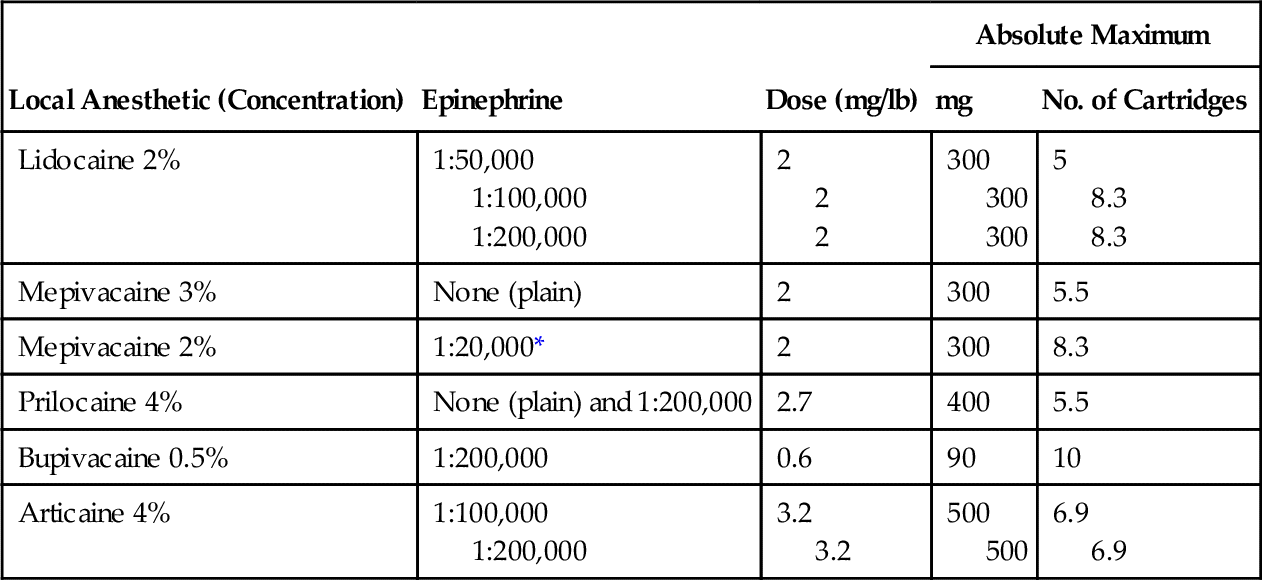
Local anesthetic agents are divided chemically into two major groups: the esters and the amides (Table 9-1). A few agents fall outside these two groups and are called other. The clinical importance of this division is associated with potential allergic reactions. A patient who has an allergy to one group is more likely to exhibit a hypersensitivity reaction to other agents within the same group. Cross-hypersensitivity between the amides and the esters is unlikely. The structure of local anesthetics is composed of the following three parts:
The aromatic nucleus (R) is lipophilic (lipid soluble), and the amino group is hydrophilic (water soluble). The esters are largely metabolized in the plasma, and the amides in the liver.
Mechanism of action
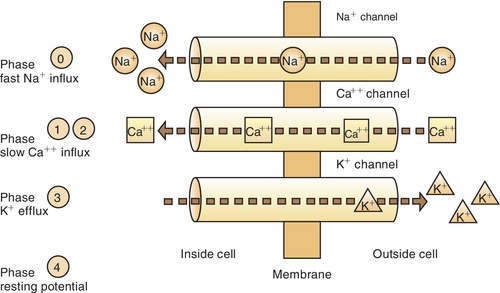
Action on Nerve Fibers
A resting nerve fiber has a large number of positive ions (cations) on the outside (electropositive) and a large number of negative ions (anions) on the inside (electronegative). The nerve action potential results in the opening of the sodium (Na+) channels and an inward flux of sodium, resulting in a change from the − 90-mV potential to a + 40-mV potential (Figure 9-3 and Box 9-2). The outward flow of potassium (K+) ions repolarizes the membrane and closes the sodium channels. Local anesthetics attach themselves to specific receptors in the nerve membrane. After combining with the receptor, the local anesthetics block conduction of nerve impulses (thus the term nerve block) by decreasing the permeability of the nerve cell membrane to sodium ions. This then decreases the rate of depolarization of the nerve membrane, increases the threshold for excitability, and prevents the propagation of the action potential. Local anesthetics may reduce permeability by competing with calcium (Ca++) for the membrane binding sites and by preventing the onset of nerve conduction.
Ionization Factors
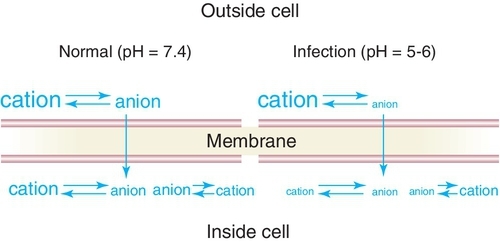
The local anesthetic agents are weak bases occurring in equilibrium between their two forms, which are the fat-soluble (lipophilic) free base and the water-soluble (hydrophilic) hydrochloride salt (Figure 9-4). Table 9-2 provides an overview of the characteristics of the lipophilic free base and water-soluble hydrochloride salt forms of the local anesthetics. The proportion of drug in each form is determined by the pKa—the pH at which half is in each form (salt and base equal)—of the local anesthetic and the pH of the environment. Local anesthetics without a vasoconstrictor range in pH from 5 to greater than 6. Local anesthetics with a vasoconstrictor range in pH from approximately 3 to 5 because of the addition of the preservative sodium bisulfate. In the acidic pH of the dental cartridge (4.5), the proportion of the drug in the ionized form rises, thereby increasing solubility. Once injected into the tissues (pH 7.4), the amount of local anesthetic in the free-base form increases. This provides for greater tissue (lipid) penetration (Figure 9-5). In the presence of an acidic environment, such as infection or inflammation (pH lower), the amount of free base is reduced (more in ionized form), one reason that dental anesthesia with a local anesthetic is more difficult when infection is present. Other reasons include dilution by fluid, inflammation, and vasodilation in the area. Although the free-base form is needed to penetrate the nerve membrane, it is the cationic form that exerts blocking action by binding to the specific receptor site.
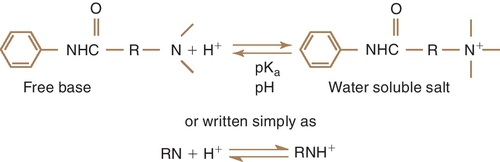
Table 9-2
Propertiesof Base and Salt Forms of Local Anesthetics
| Free Base | Salt |
| Viscid liquids or amorphous solids | Crystalline Solids |
| Fat soluble (lipophilic) | Water soluble (hydrophilic) |
| Unstable | Stable |
| Alkaline | Acidic |
| Uncharged, nonionized | Charged, cation (ionized) |
| Penetrates nerve tissue | Active form at site of action |
| Form present in tissue (pH 7.4) | Form present in dental cartridge (pH 4.5-6.0) |
Pharmacokinetics
Absorption
The absorption of a local anesthetic depends on its route. When it is injected into the tissues, the rate of absorption depends on the vascularity of the tissues. This is a function of the degree of inflammation present, the vasodilating properties of the local anesthetic agent, the presence of heat, and the use of massage. It is important to reduce the systemic absorption of a local anesthetic when it is used in dentistry. With lower systemic absorption, the chance of systemic toxicity is reduced. To reduce absorption, a vasoconstrictor is added to the local anesthetic. The vasoconstrictor reduces the blood supply to the area, limits systemic absorption, and reduces systemic toxicity.
With topical application, especially on the mucous membranes or if the surface is denuded, absorption can approximate that produced by intravenous (IV) injection. Absorption is also determined by the proportion of the agent present in the free-base form (nonionized).
Distribution
After absorption, local anesthetics are distributed throughout the body. Highly vascular organs have higher concentrations of anesthetics. Local anesthetics cross the placenta and blood-brain barrier. The lipid solubility of a particular anesthetic affects the potency of the agent. For example, bupivacaine, used as a 0.5% solution, is about 10 times more lipid soluble than lidocaine used as a 2% solution.
Metabolism
The local anesthetic agents are metabolized differently, depending on whether they are amides or esters. Esters are hydrolyzed by plasma pseudocholinesterases and liver esterases. Procaine is hydrolyzed to p-aminobenzoic acid (PABA), a metabolite that may be responsible for its allergic reactions. Some patients who have an atypical form of pseudocholinesterase that does not allow them to hydrolyze these esters may exhibit an increase in systemic toxicity if given an ester.
Amide local anesthetics are metabolized primarily by the liver. In severe liver disease or with alcoholism, amides may accumulate and produce systemic toxicity. A small amount of prilocaine is metabolized to orthotoluidine, which can produce methemoglobinemia if given in very large doses. By reducing hepatic blood flow, cimetidine can interfere with the metabolism of the amides. (This is usually unimportant in dentistry because only one dose is given. No accumulation can result if repeated doses are not administered.)
Excretion
The metabolites and some unchanged drug of both esters and amides are excreted by the kidneys. With end-stage renal disease, both the parent drug and its metabolites can accumulate.
Pharmacologic effects
Peripheral Nerve Conduction (Blocker)
The main clinical effect of the local anesthetics is reversible blockage of peripheral nerve conduction. These agents inhibit the movement of the nerve impulse along the fibers, at sensory endings, at myoneural junctions, and at synapses. Therefore they may have wide-reaching effects on many kinds of nerves. Because they do not penetrate the myelin sheath, they affect the myelinated fibers only at the nodes of Ranvier. The local anesthetics affect the small, unmyelinated fibers first and the large, heavily myelinated fibers last. This pattern is probably related to the ability of these agents to penetrate to their sites of action.
The losses of nerve function are listed in Box 9-3. The list is in the order in which the senses are typically lost, but some individual variation occurs among patients. In some patients, the pain sensation is lost before the cold sensation. The functions of the individual nerves return in reverse order.
Antiarrhythmic
Local anesthetics have a direct effect on the cardiac muscle by blocking cardiac sodium channels and depressing abnormal cardiac pacemaker activity, excitability, and conduction. They also depress the strength of cardiac contraction and produce arteriolar dilation, leading to hypotension. These properties make them useful to be given intravenously in the treatment of arrhythmias.
Adverse reactions
The adverse reactions and toxicity of the local anesthetics are directly related to the plasma level of drug.
Considering the widespread use of these agents, their potential for danger must be minimal. Deaths from local anesthetics are difficult to document, but dental treatment-related mortality is even rarer. Table 9-3 lists the maximal safe doses for common local anesthetics. Factors that influence toxicity include the following:
Children, elderly individuals, and debilitated persons are more susceptible to the adverse reactions of the local anesthetic agents. The symptoms of an overdose of the local anesthetic agents are directly proportional to the blood level attained.
Toxicity
The two main systems affected by local anesthetic toxicity are the CNS and the cardiovascular system.
Central Nervous System Effects
CNS stimulation may occur before CNS depression. CNS stimulation due to depression of the inhibitory fibers results in restlessness, tremors, and convulsions. CNS depression due to depression of both the inhibitory and facilitative fibers results in respiratory and cardiovascular depression, and coma follows.
Cardiovascular Effects
The local anesthetic agents can produce myocardial depression and cardiac arrest with peripheral vasodilation. The usual concentrations that are achieved with administration of dental anesthesia would not be expected to result in any of these adverse reactions, although deaths have been reported with the use of lower doses of anesthetic. It is postulated that the effect of these agents on heart conduction may cause a fatal arrhythmia.
Local Effects
Local effects can occur with the administration of local anesthetic agents. This is most commonly the result of physical injury caused by the injection technique or the administration of an excessive volume too quickly to be accepted by the tissues. Occasionally, a hematoma may result.
Malignant Hyperthermia
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses