Fig. 2.1
A powder/liquid RM-GIC lining material. The scoop and bottle tip have been specifically designed for providing the correct powder/liquid ratio, but it is also important to ensure the powder has been ‘fluffed up’ in the bottle prior to filling the scoop
Due to the inconsistency of mix caused by dispensing the powder and liquid separately, the effect of varying mixing time and environmental influences such as ambient temperature and relative humidity, manufacturers developed capsulated GICs for both conventional and resin-modified materials. This provided practitioners with a high level of consistency of mix, the ideal viscosity for insertion into cavities and best physical and aesthetic properties and reduced the effects of temperature and humidity. Typically, each manufacturer has developed its own capsule design (Figs. 2.2 and 2.3), but essentially the method of use is very similar, i.e. activation of the capsule followed by mechanical mixing. The one great advantage of capsule use is the ease of inserting the viscous cement into cavity preparations in almost any part of the oral cavity.
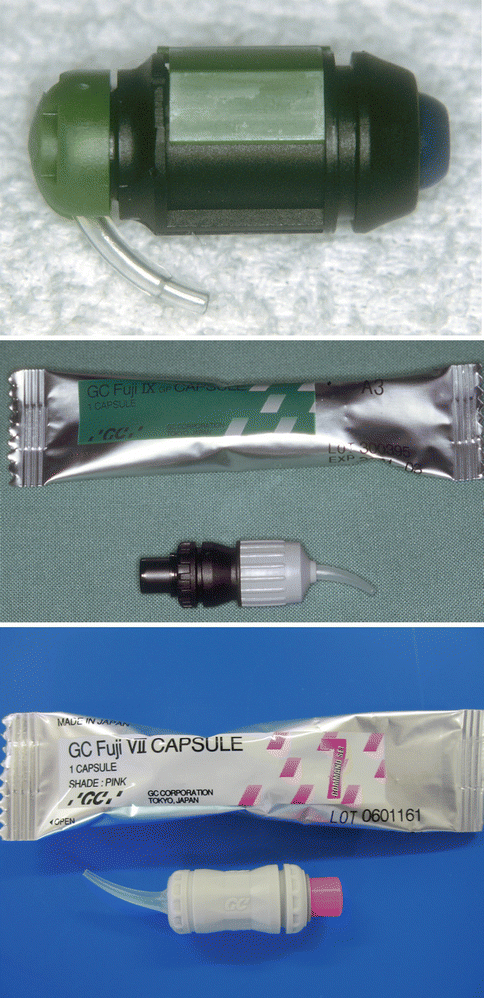
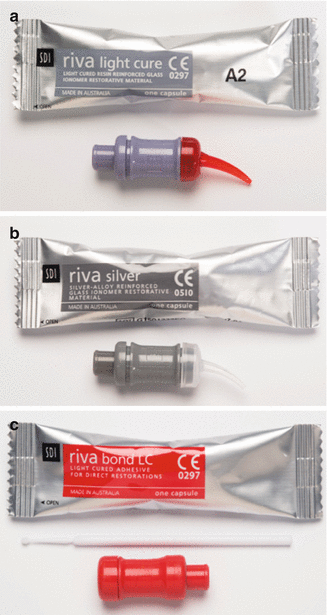

Fig. 2.2
Various types of capsules used for GIC materials

Fig. 2.3
Three different types of GICs delivered in capsule form: (a) a resin-modified GIC, (b) a silver-reinforced GIC and (c) a resin-modified GIC adhesive. Note that the last material is much more fluid and is applied to the tooth surface as a thin film to bond resin composite to the tooth surface. The material is mixed in an amalgamator which ensures a good consistency due to the manufacturer-controlled powder/liquid ratio
More recently, there has been a further development by manufacturers to develop paste/paste systems. This is especially useful for those materials that are used as a thin lining over a cavity surface. The GIC is usually a RM-GIC but can be light-cured or chemically (self-)cured. By having a paste/paste system, again the best physical properties can be attained as well as being able to be dispensed in small quantities. Each manufacturer has developed its own system with some of those materials that were originally dispensed as a powder and liquid being modified to a paste/paste system, making it simpler to mix and use (Figs. 2.4 and 2.5).
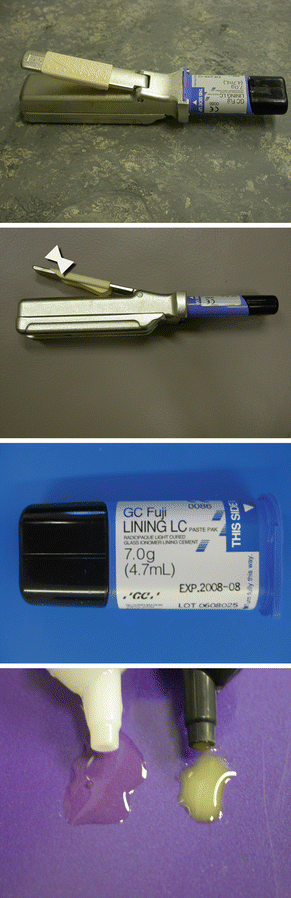
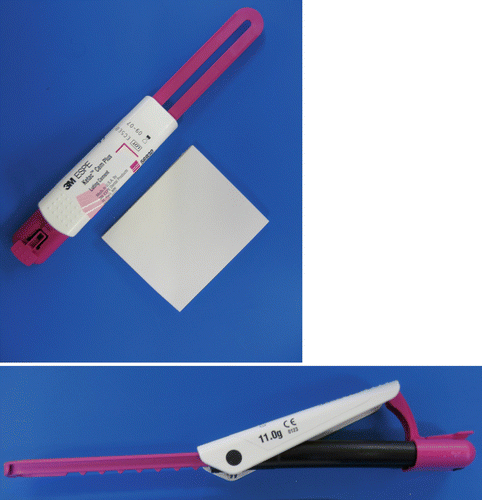

Fig. 2.4
Paste/paste dispenser for an RM-GIC lining material. Small equal amounts can be easily dispensed and mixed for placement in a small cavity. The quantity of material is varied by sliding the cream-coloured sleeve up and down the handle

Fig. 2.5
Another type of RM-GIC paste/paste dispensing system. A ‘clicker’ system is used to dispense equal amounts of the paste from each half of the tubes
2.3.1 Setting Reaction
The setting of GICs is via the attack of the glass filler particles by the acid liquid. Surface dissolution of glass particles releases metal ions such as calcium, strontium and aluminium into the newly created matrix which is formed by cross-linking with the polyacid (Cook 1983). This setting reaction is dependent on the component parts of the powder and liquid.
The filler portion is made up of a fluoroaluminosilicate glass which can range from 40 to 75 % by weight in the cement mix. The proportion of filler relates to the qualities required for the cement, for example, low-viscosity luting cements or fissure protection materials have less powder compared with high-strength and high-viscosity cements used as restorative materials that are likely to bear occlusal loading (Frankenberger et al. 1997). The set cement becomes a composite comprising unreacted glass fillers which are surrounded by a siliceous gel which is embedded in a matrix made up of the polyacid salt that is responsible for holding the set cement together (Watson et al. 2014). The component parts of the cement are described in more detail below.
2.3.1.1 Glass
The glass particles in GICs are more reactive than those found in resin composite materials. This has been achieved by incorporating fluorine into the glass. The original glass was based on the composition of SiO2–AlO3–AlPO4–NaAlF6 (Smith 1998). The early work by Wilson and McLean (1988) showed a ratio of 1:2 (or more) of Al2O3/SiO2, and fluoride of up to 23 % was required for a viable GIC.
The original work when developing GICs was to use a glass made of calcium, fluorine, aluminium, silicon and oxygen. Further developments substituted the calcium with strontium and more recently zinc. Essentially, all GIC glasses have been based on a similar formula of calcium or strontium fluoroaluminosilicate glass (Shin-ichi et al. 2000; Zimehl and Hannig 2000; Nicholson 1998). The cements also contain other ions such as sodium, phosphorus, lanthanum, barium, boron and zinc. Strontium has been used to replace calcium and lanthanum to partially replace the aluminium, which gives the cement greater radiopacity. The composition of glasses has been extensively investigated but is beyond the scope of this book (De Barra and Hill 1998, 2000; Griffin and Hill 1999, 2000a, b). It would seem, however, that the Al:Si ratio is important to the glass composition, and this may influence the fluoride release (Akinmade and Nicholson 1994; Griffin and Hill 1999).
The glass in glass-ionomer has an amorphous structure in which microcrystalline structures seem to be present. The work by Schwieger et al. (2000)) using a model of the GIC setting reaction showed that the CaF2 phase seemed to be preferentially leached to establish a silicon-rich surface on the glass which was greater than the size of the glass particles.
The inclusion of fluorine in GICs was not originally to impart an anticariogenic effect, but to aid the setting reaction of the GIC. It acts as a network disrupter allowing ion release necessary for the setting reaction. The final cement is formed by the cross-linking of the polyalkenoate matrix with strontium, calcium, aluminium and lanthanum ions. Silicon tends to remain nonreactive in the GIC reaction; it gives strength and stability to the set cement.
A recent paper showed that when the particle size of the glass is reduced, the reactivity of the particles is greatly increased with the same glass composition (De Caluwé et al. 2014). This also tends to decrease the setting time when ‘nanogranular’ particles replace the ‘macrogranular particles’. Additional fluoride in the glass tends to decrease the setting time (De Caluwé et al 2014). The compressive strength increases as more nanogranular particles containing more fluoride are used.
2.3.1.2 Liquid
The other important component of the GIC is the liquid. The liquid is a polymeric acid having a carboxylate group(s), but it must be a lower molecular weight to prevent gelation. There are a broad range of acids that can be used, and each will provide some (Smith 1998) variation in the potential application of the set GIC (Mount 1990). The liquid can also contain water or tartaric acid. In addition, by increasing the number of carboxyl groups into the polymer chains, it also helps prevent gelation during setting, which imparts greater reactivity due to a greater number of carboxyl groups. This may also lead to increased cross-linking, thus enhancing the properties of the cement such as strength (Smith 1998). It appeared that larger-molecular-weight acids seemed to improve the overall physical strength of the cement (compressive strength, fracture toughness, etc.) (Fennel and Hill 2001a, b, c). In general, however, to achieve the ideal properties of the cement, some compromise must be made to the concentration of the liquid, which is usually limited to 50 % w/w.
The role of tartaric acid was investigated as the GICs were just starting to emerge as a clinically useful material (Wilson et al. 1976; Crisp and Wilson 1976). The inclusion of tartaric acid was shown to initially reduce the viscosity of the cement then rapidly increase it, almost leading to a ‘snap’ set (Prosser et al. 1982a, b). It seems that this action is due to the chelation of ions from the glass powder over the short term, which delays the formation of the gel stage of the cement. This leads to faster cross-linking of the polyacrylic acid component (Nicholson et al. 1988); hence, most GICs contain tartaric acid to improve the working time but reduce the setting time (Young et al. 2000).
It has also been shown that water is an important component in the setting reaction of GICs. It seems that the primary role of the water is to influence the acid–base reaction. The carboxylate groups dissociate allowing them to become active for ion transfer, and the water provides the medium for ion movement to the glass powder surface (Prentice 2005).
During the setting of the GIC, the amount of bound water in the matrix also increases. The matrix contains a degree of unbound water, hence the necessity for avoiding dehydration or desiccation (Small et al. 1998). This water transfer affords GICs the advantage of movement of ions (such as fluoride ions) that may reduce the damage caused in early caries attack. There is also some evidence indicating that ion uptake may occur from saliva leading to a surface hardening of the set cement surface (Okada et al. 2001).
2.3.2 Strength
Set glass-ionomer cements tend to be brittle materials. The final strength can be affected by a number of variables. The inclusion of resin into the cement seems to show improved strength but also tends to make the cement slightly less brittle in nature. This brittleness is one of the major reasons for GICs not being well suited for larger posterior load-bearing restorations.
During setting, GICs are sensitive to both moisture loss and uptake. Loss of water leads to dehydration of the cement that causes subsequent surface crazing and increased opacity. The consequences can thus be a weaker cement, a decrease in wear resistance and a loss of aesthetics. Recently, the high powder/liquid (P:L) ratio cements have shown some improvement in water sensitivity. These cements need to be protected during the 2 to 7 min of the initial set, depending on the product and whether it has been classified as a ‘fast’ or ‘normal’ set material. After this initial set, the high P:L ratio materials (e.g. Fuji IX GP, Fuji IX GP Fast, Ketac Molar, Ketac Molar Quick, Riva Self Cure Regular/Fast) can be trimmed under water spray without loss of strength or aesthetics. For the original conventional GICs, it was necessary to coat the cement with a waterproof material to prevent water uptake and weakening of the setting cement for 24 h. It would seem that over time, the physical properties of conventional GICs tend to improve. The recent work by Shiozawa et al (2014) showed that five different GICs all tended to show an initial increase in compressive strength and surface hardness over the first 1–3 months of storage in deionised water. They then remained reasonably constant although slight decreases were observed (Table 2.1); this was product dependent. They showed that the surface quantities of Si, Sr, Na and F decreased over the period of 1 year. This demonstrated that there was some decrease in the surface integrity and hardness of the cements due to storage in the water. The decrease in surface hardness is contrary to that found by Okada et al (2001), who stored their samples in saliva and distilled water and used a time period limited to 40 days. This is equivalent to the early times noted for the increase in strength and hardness in Shiozawa et al.’s (2014) study. It can be useful to further assess the phenomenon of hardness or microhardness as it may have clinical implications for restorations subjected to occlusal loading. It can give some indication of the overall strength of the cement as well as surface changes when exposed to the various fluids GICs come in contact with during function.
Table 2.1
Examples of flexural and compressive strengths of glass-ionomer materials
|
Material
|
Type of GIC
|
Flexural strength (MPa)
|
Compressive strength (MPa)
|
|---|---|---|---|
|
Miracle Mix
|
Metal-reinforced
|
45
|
117 (21d)
|
|
Ketac Silver
|
Metal-reinforced
|
22.9 (7d)
|
211 (7d), 127 (21d)
|
|
Ketac Molar
|
Conventional high P:L ratio
|
44.1, 34.5 (24 h), 21.2 (7d)
|
86.2 (1 h), 177 (24 h), 232 (24 h), 301 (7d), 184 (21d)
|
|
Fuji IX/ Fuji IX Extra
|
Conventional high P:L ratio
|
33.3, (24 h), 20.2 (24 h)
|
99.5 (1 h), 83.3 (1 h), 166.7 (24 h), 201 (24 h), 168 (21d)
|
|
Riva
|
Conventional
|
23.9 (24 h)
|
126.5 (24 h), 200 (24 h)
|
|
Photac-Fil
|
Resin-modified
|
74.4 (7d)
|
243.5 (7d), 150 (21d)
|
|
Fuji II LC
|
Resin-modified
|
42.1, 49.6 (24 h), 71.1 (7d)
|
306 (7d), 166 (21d)
|
Interestingly, one study examined Knoop hardness of a high-viscosity GIC that had been harvested from 10-year-old restorations (Zanata et al. 2011). The hardness was compared with the same material stored in water for up to 720 days. The outcomes for this study showed a similar increase in hardness which was no different from the laboratory-stored samples. The 10-year-old samples were similar in hardness to the water-stored specimens after 180 days. Energy dispersive X-ray diffraction (EDX) analysis showed that Ca was present in the cement which seemed to indicate that Ca from the oral cavity was able to diffuse into the cement.
The introduction of RM-GICs helped to overcome some of the initial dehydration problems of the conventional GICs. However, once set, the RM-GICs also show moisture sensitivity. If allowed to dehydrate, the surface crazes and becomes opaque. Hence, it is critical to maintain the water balance within the matrix of all GICs to ensure that the maximum strength possible is ensured as well as the best aesthetics.
One of the commonly mistaken concepts is that GICs do not shrink (Kim and Hirano 1999; Bryant and Mahler 2007). Both conventional GIC and RM-GIC shrink during setting. The shrinkage from the acid–base reaction portion of the set is slower, but not necessarily less than the shrinkage of the resin portion. In RM-GICs, shrinkage occurs more rapidly during the light-curing phase (Cheetham et al. 2014). In this case, the shrinkage from the acid–base component is minimal.
When it comes to the stress of the bond to the tooth, it is likely that GICs can resist some of these forces better when a resin composite restoration is placed. The cement goes through a rubbery gel stage during its set. This may assist with countering some of the stresses occurring from a light-polymerising resin composite which can be rapid and high depending on the type of composite used.
2.3.3 Fracture Toughness
As GICs are regarded as brittle and failure is related to material fracture, some researchers have focused on the fracture toughness of these and other tooth-coloured filling materials. This approach helps to characterise fracture resistance and provides some indication as to how much energy of loading is required to cause a material to fail. Several other studies have looked at this characteristic. In an approach to improve fracture toughness of GICs, resin coatings have been applied to the surface of GICs. Both conventional and resin-modified materials have been tested. It was previously shown that the application of a resin coating on the resin-modified materials generally showed an increase in toughness (Mitchell et al. 1999; Mitsuhashi et al. 2003; Ilie et al. 2012). In comparison with resin composite materials, several studies have shown that the conventional materials have the lowest fracture toughness, whereas the RM-GICs have ‘comparable toughness’ to the microfilled, flowable and nano-hybrid resin composites (Ilie et al. 2012) (Table 2.2).
Table 2.2
Fracture toughness of various GICs
|
Material
|
Type of GIC
|
Fracture toughness (K1C, MPa m1/2)
|
|---|---|---|
|
ChemFil Rock
|
Zinc-reinforced
|
0.99
|
|
Fuji IX GP Extra/Fuji IX
|
Packable
|
0.8/0.53
|
|
Ketac Molar Quick Aplicap/Ketac Molar
|
Packable
|
0.85/0.48
|
|
EQUIA Fil
|
Resin-coated
|
1.21
|
|
Ketac Silver
|
Silver-reinforced (Cermet)
|
0.44
|
|
Ketac Fil
|
GIC
|
0.39
|
|
Fuji II LC
|
RM-GIC
|
1.16
|
|
Photac-Fil
|
RM-GIC
|
1.32
|
Mitsuhashi et al. (2003) showed that there was a high correlation between the P:L ratio of conventional GICs and fracture toughness, whereas this pattern was not observed for RM-GICs. It seems that the resin component is able to increase toughness and perhaps fill in spaces where a crack may propagate more easily. One of the problems for fracture toughness measurement and comparison amongst research groups, however, is the test methodology used. The test method can lead to different outcomes as seen in Table 2.2.
2.3.4 Shear Punch Strength
Shear punch strength is another test method that has been used for comparison of materials and evaluation of coatings of GICs. This method is quite simple and gives some indication of how a cement behaves when it is loaded during function (Nomoto et al. 2001). Although not widely used, it has shown some interesting outcomes. The first shear punch strength evaluation was published in 1996 (Mount et al. 1996). This comprehensive study included conventional GIC and RM-GIC as well as a number of resin composite materials. The study was interesting in that it investigated 2-h strengths compared with 5-day strengths. They showed that for the conventional GICs, the strength showed a significant increase from the 2-h test to that of 5 days. In the case of the RM-GICs, which could be either light- or auto-cured, again it was noted that the strength increased significantly over the 5 days. If allowed to only auto-cure (i.e. no light exposure), the strength tended to be less at 5 days; hence, it would seem the light-curing aspect to curing is an essential step to achieve maximum strength. In fact, the Photac-Fil material (3M-ESPE, St Paul, MN, USA) tested at 5 days showed a lower strength than the 2-h light-cured strength. For the resin composite materials, the strength tended to increase by about 5–10 %, depending on the material, over the 5 days (Mount et al. 1996). This study was conducted prior to the introduction of the high-viscosity/high P:L ratio cements. Two later studies compared the strengths of GICs with and without resin coating (Bonifácio et al. 2012; Bagheri et al. 2013). These studies showed that in some cases, the coating was beneficial, whereas for other materials, there was little change. The interesting outcome from the study by Bagheri et al. (2013), where strengths were tested at 24 h, 4 and 8 weeks, was that irrespective of resin coating, the shear punch strengths were all observed to steadily increase over the 8 weeks of the study. In most materials, the shear punch strengths almost doubled. The effect of the coating seemed to be greater at the longer time periods after the cement had matured.
The influence of food-simulating solutions on the shear punch strength has also been evaluated (Bagheri et al. 2007; Kaur and Nandlal 2013). The solutions used were lactic acid, NaOH and coffee in one study (Bagheri et al. 2007) and citric acid, ethanol and heptane in another (Kaur and Nandlal 2013). In the first study, a RM-GIC was compared with other resin-based restorative materials, whilst in the latter study, a high-viscosity conventional GIC was assessed. The RM-GIC was shown to be strongly affected by the food-simulating solutions compared with the other resin-based materials. The same effect was also noted for the conventional GIC. It would therefore seem that GICs are more susceptible to the influence of various dietary solutions of varying pH, and this may result in some surface deterioration and weakening during clinical service.
Due to the brittle nature of GICs and their early sensitivity to water, an additional step is needed to protect the materials from water exposure in order to achieve their ‘highest’ strength. It was noted that the original cements had a soft ‘opaque’ surface layer if exposed to water too early; this was easily abraded and was quite unaesthetic (Norman et al. 1969). Other reports suggest that the newer high-strength conventional GICs may benefit from early water exposure (Leirskar et al. 2003; Wang et al. 2006).
2.3.5 Erosion
One of the important properties any restorative material must have is the ability to resist degradation from exposure to various fluids that will contact the set material in the oral cavity. The oral environment is very harsh, with restorative materials being exposed to a wide variation of temperatures and changes in acidity and alkalinity. In recent years, the loss of tooth structure due to erosive or acidic materials has become a significant issue (Kitasako et al. 2015). The matrix of the GIC is the most susceptible part of the set cement when exposed to acids. Acids such as acetic, citric and lactic have all been used to evaluate erosion (Crisp et al. 1980; Matsuya et al. 1984; Fukuzawa et al. 1987). One of the important aspects for preventing the effects of erosion is when laminate or sandwich restorations, which fill the gingival portion of deep posterior approximal restorations, are placed (van Dijken et al. 1999). The study by Scholtanus and Huysmans (2007) showed the erosive degradation of approximal lesions restored with a GIC.
As noted above, the conventional GICs are sensitive to water exposure shortly after placement. The water will damage and erode the surface of the GIC if it is left unprotected on insertion (Oilo 1984; Gemalmaz et al. 1998).
In the case of sandwich/laminate restorations, if a patient’s oral hygiene is not adequate, then the biofilm may produce acid which can damage the surface of the set cement. This problem is exacerbated when the quantity of saliva is compromised and does not wash the acids away or have adequate buffering capacity. It has been shown (Nicholson et al 2000), however, that when GICs are exposed to lactic acid, the surrounding pH decreases initially, but as the GIC dissolves, the pH increases. Hence, GICs seem to exhibit a ‘side effect’ of being able to reduce the effects of acid attack by their own dissolution (Nicholson et al 2000). The Scholtanus and Huysmans (2007) study showed that this dissolution of conventional GIC has the beneficial effect of preventing caries initiation on susceptible adjacent tooth surfaces. It is most likely due to the constant exposure of a ‘fresh’ GIC surface that is able to release ‘maximum’ levels of fluoride ions into the surrounding environment and tooth structure. When erosion does occur, it is the matrix of the set cement that is most susceptible to damage (De Moor and Verveeck 1998; Patel et al. 2000).
RM-GICs are also susceptible to erosion. Water has been demonstrated to have an erosive effect on the surface of RM-GICs (Cattani-Lorente et al. 1999; Fano et al. 2004). It would appear that the amount of light irradiation, i.e. the duration of light curing, may have an influence on the degree of erosion of RM-GICs (Fano et al. 2004). They showed that an exposure time of less than 15 s resulted in cracking of the cement and a greater degree of erosion. The same study showed that the pH of the immersion solution also influenced the degree of erosion, which was also noted in the study by Czarnecka and Nicholson (2006). However, like the conventional GICs, when the degree of erosion does increase, the release of fluoride also increases (Carey et al. 2003), thus affording some benefit to assist with controlling demineralisation around cavity margins and adjacent teeth.
A recent study using pH cycling over a 35-day period using a cola drink and artificial saliva showed that both the conventional GIC and RM-GIC displayed greater amounts of erosion compared with amalgam and resin composite (Honório et al. 2008).
A study investigated the effects of erosion of a resin composite, conventional GIC and RM-GIC placed into root dentine cavities (Soares et al. 2012). It was observed that the acid erosion severely degraded the GIC surfaces but afforded the dentine at the cavity margins some protection against the erosive solution due to the ions released from the degrading GIC (Soares et al. 2012). Ion release has also been demonstrated in the study by Zalizniak et al. (2013) where GICs were exposed to various acid solutions. It was observed that ion release, particularly phosphate ions, seemed to be dependent on the type of acid the GICs were exposed to. The mechanism is still not well understood and needs further investigation.
Erosion also affects surface roughness. A study comparing resin composite and conventional GIC and RM-GIC showed large differences in surface roughness. Hence, it would seem that the addition of resin into RM-GICs may not provide a long-term benefit from the aspect of surface finish when exposed to an acidic solution (Hussein et al. 2014).
One of the areas which has so far not been investigated to any great extent is the influence of the new coating agents that are now used on GICs. These coating materials are resin-based and have been shown to increase the fracture toughness of the materials (Bagheri et al. 2010). However, little research has been conducted to determine whether these resin coatings or even the placement of a coat in the form of a resin-based adhesive or bonding resin will reduce the erosion. Unfortunately, this may also cause some reduction in the release of fluoride ions that may make the GICs less effective in reducing the caries experience or recurrence around margins or on adjacent teeth (Mazzaoui et al. 2000).
2.3.6 Abrasion
Often in association with erosion is abrasion of the softened GIC surface. Compared with resin composite materials, it has been reported that GICs have a much lower abrasion resistance. It is also known that due to the maturation of GICs during the setting process, their abrasion resistance is poor compared to the fully matured cement (Mount and Hume 2005). Although not aesthetic, it has been shown that the metal-reinforced GICs have a better abrasion resistance (Forss et al. 1991). With the introduction of the high powder/liquid ratio and small particle materials such as Fuji IX (GC Corp, Tokyo, Japan) or Ketac Molar (3M-ESPE, St Paul, MN, USA), it has been demonstrated that the wear (abrasion) can be reduced significantly (Kunzelmann et al. 2003).
Another means to increase abrasion resistance is also the concept of coating the GIC. To date, the research remains limited, similar to that for erosion resistance. One study using the resin glazing agent Bellfeel Brightener (Kanebo Ltd, Tokyo, Japan) when applied to a GIC surface showed a significant increase in surface hardness and thus more resistance to abrasion (Hotta and Hirukawa 1994). More recently, the use of proprietary resin coating agents, e.g. G-Coat Plus (GC Corp, Japan) in combination with the high-viscosity GIC, Fuji IX, has been marketed as EQUIA (GC Corp, Japan) or more recently as EQUIA Forte Fil (GC Corp). The application of the coating as described previously has been shown to increase the strength of the GIC and increase its abrasion resistance. It is believed the resin is able to infiltrate the GIC surface, thus filling cracks and porosities (Lohbauer et al. 2011).
For the RM-GICs, the incorporation of the resin was not shown to improve the abrasion resistance. In fact, a number of studies have reported that the abrasion resistance is decreased and that the RM-GIC materials will abrade more rapidly than conventional GICs (Pelka et al. 1996; Momoi et al. 1997; Peutzfeldt et al. 1997; Xie et al. 2000; Sunnegårdh-Grönberg et al. 2002). The reason for this reduction in abrasion resistance is thought to be due to the glass particles being bonded loosely to the matrix in association with a nonuniform distribution of the glass particles throughout the set cement (Xie et al. 2000). When a polyacid-modified resin composite (PAMRC) was compared with an RM-GIC clinically, it was also noted that the abrasion resistance was lower for the RM-GIC (Chinelatti et al. 2004).
2.3.7 Adhesion
One of the great advantages of GICs is that they have become known for their ability to adhere to the moist cut tooth surface. This group of materials is able to bond to all parts of the tooth and carious tooth structure with a high degree of reliability and low technique sensitivity. Interestingly, however, there has not been as wide an evaluation of adhesive tests compared with resin-based adhesives. The original glass-ionomer cements were applied to smear layer-covered dentine. In the mid-1980s, workers started to consider how the adhesion of GICs might be improved (Lacefield et al. 1985). It was known from the work with phosphoric acid on enamel that adhesion for resin-based materials could be greatly enhanced. Various treatments such as polyacrylic acid, H2O2, citric acid or surface cleaning alone were tested. It was reported that the polyacrylic acid showed improved adhesion (Hinoura et al. 1986). Around 1990, manufacturers introduced conditioners such as Ketac Conditioner (3M-ESPE, St Paul, MN, USA) and GC Conditioner (GC Corp, Tokyo, Japan) for conditioning the dentine and enamel. It was shown that the use of these polyacrylic acid-based materials greatly enhanced the adhesion (Joynt et al. 1990; Tanumiharja et al. 2001). After such work, it became a routine practice to condition the dentine prior to GIC placement. These studies showed that the conditioning removed the smear layer but did not remove the smear plugs which ‘protected’ the dentine from becoming very wet. The tooth surface is not etched in the same way as acids such as phosphoric acid. This was the commencement of routine conditioning of tooth surfaces prior to placement of a GIC lining or restoration as opposed to etching for enamel and dentine with resin-based adhesives. Later work showed that removal of the smear layer could lead to better adaptation and bonding of the cement to the tooth surface. Another early study showed that this improved adhesion could be achieved with the use of maleic acid conditioning of dentine prior to the placement of Vitrebond (3M-ESPE, St Paul, MN, USA), which was one of the first resin-modified GICs to become available commercially (Watson 1990). Later Tyas showed that the use of polyacrylic acid conditioning clinically seemed to have little effect on restoration survival (Tyas 1993). This same outcome was shown in another study comparing 10 % polyacrylic acid conditioned and nonconditioned non-carious cervical lesions over a 4-year period. However, a slightly better retention rate was observed for the conditioned group, 15.6 % loss compared with 21.9 % loss in the nonconditioned group (van Dijken 1996a). Slightly later, a further study investigated the adhesion of a ‘viscous’ conventional GIC, Chem-Flex (Dentsply DeTrey GmbH, Konstanz, Germany), to dentine using different conditioning agents. These included 10 % polyacrylic acid (PAA) without rinsing, 10 % PAA with water rinsing, 25 % PAA with rinsing and 32 % phosphoric acid and a control of smear layer-covered dentine (Tay et al. 2001). Further to the bond study, this group also investigated the interface using transmission electron microscopy (TEM). It was shown that bond strengths after conditioning were much higher compared to the control. The TEM observations showed that the acidity of the GIC during setting was not able to alter the smear layer-covered control surface. However, when 10 % PAA was used as the conditioning agent, it was observed that the smear layer was removed and also partial demineralisation occurred up to 0.8 μm deep. An ‘interphase’ was noted between the tooth surface and cement (Tay et al. 2001). With the stronger acids or longer conditioning times, the depth of demineralisation increased. This interphase layer was also reported in the study by Tanumiharja et al. (2001), who investigated the interface using field emission scanning electron microscopy. They observed that this layer was resistant to attack by acidic and basic solutions and could range between 2.8 and 3.4 μm thick. A very similar phenomenon was noted when an RM-GIC was bonded to conditioned dentine. This study showed that using a conditioner provided a significant improvement in bond strengths. The interface of the RM-GIC and dentine also showed the acid–base resistant layer described by Tanumiharja et al. (2001). Subsequently, others observed that a hybrid-like layer similar to that formed by resin-based adhesives also formed (Cardoso et al. 2010).
With respect to RM-GICs, Coutinho et al. (2007) investigated several RM-GIC materials including an RM-GIC adhesive (Fuji Bond LC, GC Corp, Tokyo, Japan) as well as direct restorative materials (Photac-Fil and Vitrebond, 3M-ESPE, St Paul, MN, USA). They characterised the interfaces using transmission, scanning and field emission electron microscopy and atomic force microscopy. The tooth surfaces were either not conditioned or conditioned and treated with either a 20 % acrylic–maleic acid copolymer or 25 % polyacrylic acid. For the Fuji Bond LC conditioned samples, a very thin gel phase layer (0.5–1.0 μm thick) was observed above a sub-micrometre hybrid layer (Coutinho et al. 2007). This was also noted for the Photac-Fil but not the case for Vitrebond.
When conditioning did not take place, the gel phase was not observed, but partial demineralisation still occurred as in the conditioned groups. It would seem that when RM-GICs adhere to the tooth surface, it is a dual-type adhesive process. A thin hybrid layer is formed onto a partially demineralised surface with hydroxyapatite still remaining present in the collagen fibre matrix. Above this hybrid layer is a thin gel phase layer which has been identified previously as an ‘absorption layer’ (Sidhu and Watson 1998). Coutinho et al. (2007) showed that in the case of Vitrebond, it did not react in the same manner as the other RM-GICs tested. There was no sign of a hybrid layer or gel phase formation, but it did not seem to affect the bond. The other part of the bond seemed to be due to the reaction of polycarboxylic acid copolymers interacting with hydroxyapatite to form a chemical bond (Yoshida et al. 2000). This ‘dual’ adhesion process provides an answer as to why many clinical studies show such a high success of RM-GIC restorations.
With respect to surface treatments, recently some have advocated that a short etch with phosphoric acid on dentine and enamel will enhance the bond to tooth structure when an RM-GIC is used. Whilst there may be some logic to this concept, it should be remembered that even a short etch with phosphoric acid has the potential to remove all of the hydroxyapatite from the tooth surface. Based on the work of Coutinho et al. (2007), it would appear that this would then prevent the polycarboxylate groups from interacting chemically with calcium and thus potentially remove one of the modes of adhesion of the RM-GICs, namely, the chemical portion. This may also have long-term implications on adhesion since the bond would tend to be essentially micromechanical and be subject to degradation of the collagen similar to resin-based adhesives. Recently, Hamama et al. (2014) investigated the effect of a 5-s etch with phosphoric acid compared to conditioning with polyacrylic acid-based conditioners. They showed that in the short term (24 h), the bond strengths were little different, but later unpublished work by this group showed that the bonding outcomes for the etched group tended to decrease over time or became more variable (Hamama et al., unpublished). This may be an indication that the bond using a polyacrylic acid-based conditioner can lead to a more stable bond over the long term.
2.3.7.1 Adhesion to Composite and Repair
With the advent of the sandwich or laminate technique to restore deep approximal cavities, many have questioned the ability of GICs to bond to resin composite. There is little or no problem of bonding to composite with the RM-GICs since the HEMA in the cement, being a methacrylate, can easily adhere to resin-based adhesives or resin composite, which are also methacrylate-based materials. However, concern has been expressed with respect to the ability of the resin portion of the RM-GIC to adequately polymerise in deep cavities. Although the depth of cure has been poorly studied, the few studies available seem to consistently demonstrate that depth of cure is indeed an issue that must be carefully considered. The first paper by Mount et al. (2002) concluded that ‘cavities more than 3 mm deep’ should be filled incrementally. The shade of the material was also an influencing factor for the depth of cure. A later study also made the same recommendation (Roberts et al. 2009). It would therefore seem that for cavities extending onto the root face, a conventional high powder/liquid ratio GIC is possibly the most reliable material to use. However, the issue remains of being able to achieve adhesion to the overlying resin composite.
The first study to investigate long-term adhesion of conventional GICs with recent etch-and-rinse and self-etch adhesives was that of Zhang et al. (2011). They showed that effective bonding could be achieved with any of the adhesives tested which included etch-and-rinse, self-etch and all-in-one systems. One potential issue was the effect of the phosphoric acid on the GIC surface causing microcracks that seemed to influence the long-term adhesion. The self-etch materials, however, showed quite stable adhesion over the 6 months of the test. Another study that included a conventional GIC was that of Navimipour et al. (2012). They investigated the adhesion of a conventional GIC and RM-GIC to resin composite using either phosphoric acid or Er,CR:YSGG laser for etching the GICs for 15 s. Both treatments showed improvements of bond strengths with the RM-GIC showing higher bond strengths compared with the conventional material. A further study compared RM-GIC and conventional GIC with an etch-and-rinse adhesive and acidic all-in-one adhesive (Pamir et al. 2012). This group concluded that both GICs bonded well to either adhesive, but an etch time of 30 s was recommended. The study by Zhang et al. (2011) contradicts this, as the impression was that stronger and longer etching can possibly damage the matrix of the underlying cement and weaken its cohesive strength. Generally, it was considered that the self-etch materials may provide a more reliable bond and reduce the possibility of damaging the underlying GIC. Recent studies on bonding an RM-GIC to resin composite with a variety of adhesives showed successful bonding (Kasraie et al. 2013; Boruziniat and Gharaei 2014). However, caution must still be exercised with respect to the depth of cure.
The other aspect in this section is the repair of GIC restorations. Very little work has been undertaken to determine if previously placed GIC restorations can be successfully repaired by the addition of new GIC. Only one study seems to have been published investigating the bonding of RM-GIC or resin composite to 4-day-old RM-GIC (Maneenut et al. 2010). The surfaces were treated with or without acid etch. The bond of the new RM-GIC was lower and slightly more variable in comparison with that of the resin composite. This study recommended that when addition to existing RM-GIC is warranted, the addition of resin composite is preferable (Maneenut et al. 2010). A very recent study (Welch et al. 2015) has investigated almost the same scenario as Maneenut et al. This latter study used roughening, roughening and etching or roughening, etching and the addition of a resin-based adhesive. It was concluded that the addition of the resin-based adhesive to a roughened and etched surface produced the best outcomes (Welch et al 2015). The conclusions concurred with the former study of preferably bonding resin composite to the RM-GIC when a repair is needed.
2.3.8 Ion Release
One of the major points all practitioners will mention about their reason for selection of a GIC material is the release of ions, particularly fluoride. Certainly this is a great advantage of GICs since it affords them the ability to alter the environment round the material. This allows potentially healing and/or preventing early caries attack, as well as aiding the healing of deep caries lesions in prepared cavities which have not had all the carious tissue excavated. Research is moving towards developing GICs to be able to release or supply other ions or compounds, and this is discussed elsewhere in this book. The first cements to show the effect of fluoride release and thus prevent re-initiation of caries were the silicate cements. The source of the fluoride from GICs is the glass particles. Unfortunately, it is still unclear how much fluoride is actually needed to prevent the initiation of caries. The paper by Randall and Wilson (1999) indicated that the clinical evidence was unclear with respect to the prevention of caries in teeth restored with GIC.
Some of the original work conducted on fluoride release from GICs was done by Forsten (1990, 1995). His work of 1990 was based on some of the original conventional GICs where fluoride release was evaluated from 24 h up to 8 weeks. This was the pioneering work on this important topic. It was shown that the release of fluoride was highest in the first 24 h whilst the cement was still maturing, with a large reduction in the first week, followed by a more steady decline over the 8 weeks of the study. Fluoride was still detectable at 8 weeks but at very low levels. When analysed from the aspect of cumulative release, it was noted that the amounts of fluoride detected was very much material dependent. Interestingly, Forsten also investigated the fluoride release after exposing the cements to running water for up to 22 months. Again even at 22 months, fluoride ions were still being released from the cements which were detected at about 1 part per million. The only material that did not show release of the fluoride ion was the cermet material, Ketac Silver (3 M-ESPE, USA). When exposed to an acidic solution, a greater level of fluoride was identified (Forsten 1990). Forsten followed this work up to 5 years later with an evaluation of RM-GIC fluoride release as well as uptake. This study initially observed fluoride release for up to 1 month. Again it was shown there was a high initial burst of fluoride at 24 h with a greatly reduced release by 1 month (Forsten 1995). Another aspect of the research was to determine whether 9-month-old RM-GIC specimens, which had been in running water, could take up fluoride if stored in a 50 ppm fluoride-containing solution for 1 week. He showed that even after 9 months, fluoride could still be detected, but specimens stored in the fluoride-containing solution showed a much greater release for the following week. This was probably the first paper to indicate that GICs could be ‘recharged’ with fluoride. Furthermore, it was also shown that in an acidic environment, the level of fluoride release increased (Forsten 1995). This fluoride release occurred due to erosion of the GIC, as discussed in the previous sections, where ‘fresh’ GIC that has F ions in abundance would be continually exposed. In a later review paper, Forsten noted that the fluoride release was a little different between the conventional GIC and RM-GIC. Forsten’s work also noted that the other fluoride-releasing materials such as PAMRC did not respond in the same way to the recharging process (Forsten 1998).
A more recent paper investigated the fluoride release from RM-GICs (Vitremer, 3M-ESPE; Fuji II LC, GC Corp), including an RM-GIC containing nanofillers (Ketac Nano, 3M-ESPE) and a flowable PAMRC (Dyract Flow, Dentsply), when exposed to solutions of different pHs (4, 5.5 and 7) (Moreau and Xu 2010). Fluoride release was measured up to 84 days post-setting. This study showed an initial high release of fluoride which was again material dependent. It was noted that the PAMRC and nanofilled RM-GIC released significantly less fluoride than the other two RM-GICs tested. During the first 2–3 weeks, the lower pH solutions led to greater release of fluoride, but by days 70–84, the rate of release was no different amongst the 3 different pH solutions. Figure 2.6 illustrates the typical pattern of cumulative fluoride ion release, whilst Fig. 2.7 shows the typical rate of fluoride release for these types of material.
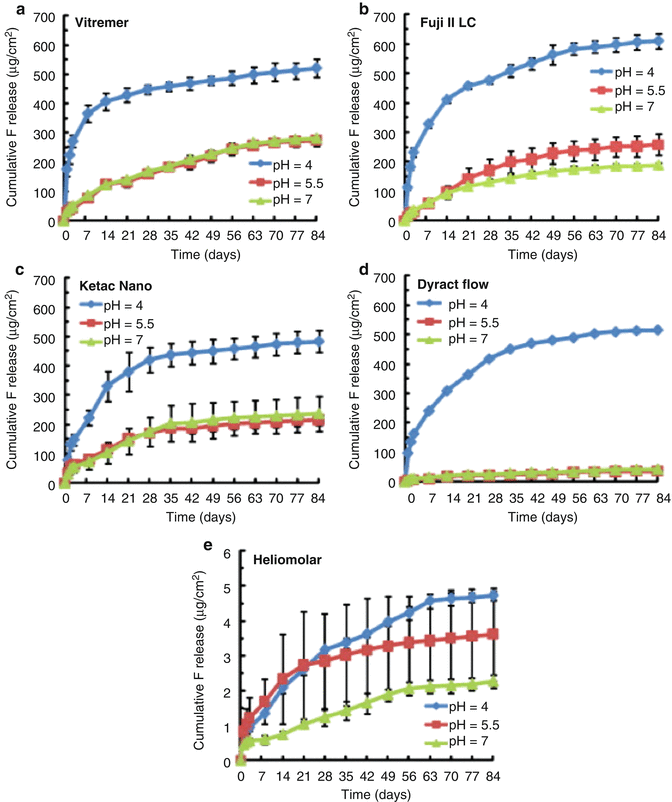

Fig. 2.6
This figure provides an illustration of the typical pattern shown for cumulative fluoride ion (F) release per specimen area (μg/cm2) from various tooth-coloured restorative materials: (a) Vitremer, (b) Fuji II LC, (c) Ketac Nano, (d) Dyract Flow and (e
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


