Fig. 4.1
The Minimum Intervention care plan framework for individualised, oral healthcare team-delivered, oral disease management (GDP general dental practitioner, OHE oral health educator, DCP dental care professional (therapist/hygienist))
This describes the overall patient-centred oral and dental healthcare delivery framework where the oral healthcare team (dentist, dental therapist, hygienist, oral health educator, nurse, practice manager and technician) collectively advises patients and offers treatment that promotes health and prevents disease. Included in this framework are disease detection and diagnosis, its non-operative (non-invasive) prevention and control, operative management where necessary, all packaged together with suitably frequent recall consultations. At these vital recall appointments (often undersold to patients by the profession using the valueless term ‘check-ups’), the patient’s motivation, behaviour and adherence to the preventive lifestyle change advised are reassessed and reinforced. This is within the context of an outcome review of the treatment provided in the previous care episode(s) (restoration status, evidence of disease progression, changes in bacterial/ionic oral balance, etc). The periodicity of these consultations must be tailored to the individual patient’s need and ongoing disease risk/susceptibility assessment.
4.1.2 Minimally Invasive (MI) Dentistry
Traditional operative caries management relies upon complete excavation of carious tissue followed by modification of the resulting cavity in terms of its surface finish and overall internal shape to aid restoration retention. The extent of this modification is dictated by the nature of the direct, plastic restorative material used to fill the cavity. Classically, for many years, non-adhesive dental amalgam has been the material of choice for most clinicians, so much so that predetermined cavity shapes are associated erroneously with GV Black’s caries classification, classes 1–5, by many dental professionals.
With improved knowledge and understanding of the patho-physiology of the caries process and the subsequent defence reactions of the dentine-pulp complex, scientific and clinical evidence now shows clearly that not all carious tissue requires excavation during interventive operative surgical procedures (Banerjee and Watson 2015). The peripheral seal of restorations with healthy enamel and dentine close to the enamel-dentine junction (EDJ) is of paramount importance to ensure the tooth-restoration complex survives (Banerjee and Watson 2015). This is in conjunction with primary preventive methods instigated by the patient to disturb and/or remove the plaque biofilm, so preventing it from stagnating and becoming cariogenic in nature. Maximum tissue preservation along with maintaining pulp vitality (sensibility) is the tenet of minimally invasive dentistry, and the development of adhesive dentistry has promoted the MI philosophy into mainstream operative care.
Glass-ionomer cements (GICs) are an important part of the contemporary restorative armamentarium for MI operative dentistry. Since their introduction by Wilson and Kent (1971), glass-ionomer cements have had a wide range of clinical uses due to their naturally adhesive, tooth-coloured nature and fluoride-leaching properties, their low coefficient of thermal expansion and ultimate biocompatibility with mineralised tissues (Burke and Lynch 1994; Olivia et al. 2000). Clinically, they have proven to be useful in restorative, lining, luting and sealing applications.
4.2 GIC Adhesion to Tooth Structure
4.2.1 Enamel
Glass-ionomer cements (GICs) bind chemically to calcium ions in hydroxyapatite (HAP), the main constituent of dental enamel. Enamel surface pre-treatment with conditioners including polyacrylic acid (PAA) improves the bond strength between enamel and GIC, based on the exchange of calcium and phosphate ions versus carboxyl ions at the enamel surface. Es-Souni et al. (1999) indicated that the improved adhesion of the GIC on polished and conditioned surfaces resulted from the combined beneficial effects of superficial surface cleanliness, better wettability and surface chemistry. They concluded that PAA conditioning of the enamel prior to GIC bonding led to the formation of a fine polymeric film on the surface. This film may act as a primer and be involved directly in the cement-building reactions, so creating a ‘stronger’ interfacial layer on the GIC aspect of the bond.
4.2.2 Fluoride and Mineralized Tissues
Bezerra et al. (2012) examined the levels of the fluoride, calcium and phosphate in the enamel and dentine alongside glass-ionomer-based restorations in vivo over time using a high-viscosity GIC (Fuji IX GP, GC Corp, Tokyo, Japan) and a resin-modified GIC (Vitremer, 3M ESPE, St Paul, MN, USA). They described a substantial increase in the fluoride ion concentration adjacent to the glass-ionomer-based restorations attributable to large differences in the ionic gradients and subsequent diffusion patterns (Ngo et al. 2006). This finding raises an important question: does the increase in fluoride ion concentrations in enamel and dentine contribute substantially to an increase in the acid resistance of these two substrates? In-vitro studies had shown an increase in the resistance to demineralization of enamel (Hatibovic-Kofman et al. 1997; Attar and Önen 2002) and dentine (Jang et al. 2001), which was attributed to the release of fluoride ions from the GIC-based restorations. Qvist et al. (2004) reported a reduction in carious lesion progression in enamel surfaces adjacent to glass-ionomer restorations in primary teeth as compared with amalgam restorations over a period of 8 years. This evidence certainly seems to indicate that the fluoride release from glass-ionomer-based materials can play a role in disease arrest in enamel and dentine that is in contact with these materials. When high-viscosity and resin-modified glass-ionomer restorations are used to restore carious lesions in primary molars using the atraumatic restorative treatment (ART) technique, fluoride ions are released into the adjacent enamel and, in particular, into the demineralized, caries-affected dentine.
4.2.3 Sound Dentine
The development of a minimally invasive adhesive approach to conservative dentistry has brought many advantages, such as preservation of tooth tissue, reinforcement/infiltration of weakened remaining tooth structure, reduced marginal leakage and the reduced potential for pulp sensitivity and maintenance of pulp vitality. Adhesive restorative materials should have a close affinity mechanically, physically and chemically to tooth tissue in a way that minimizes the risk of further ingress of bacteria and arrests disease activity. They should also have the ability to bond to a variety of overlying protective restorative materials including resin composite, metals and ceramics. One of the most attractive features of GICs is their ability to bond directly to dentine. Polyacrylate ions either react with apatite by displacing calcium and phosphate ions or bond directly to the calcium within the apatite via hydrogen bonds with the collagen and ionic bonds to the apatite within the dentine (Van Noort 2013).
There have been many studies published reporting varying bond strengths between dentine and GIC. Yip et al. (2001) measured the micro-tensile bond strength (μTBS) of three highly viscous glass-ionomer cements to sound coronal dentine; they found bond strengths in the range of 12–15 MPa, with interfacial (adhesive) and mixed modes of failure. However, previous studies (Cattani-Lorente et al. 1993; Burke and Lynch 1994; Berry and Powers 1994) suggested that bond strengths >5 MPa were seldom achieved using tensile or shear tests in vitro, with more cohesive failures occurring within the GIC (Nakajima et al. 1995). It was clear that much of the difference could be explained by variations in the experimental testing technique used, the inconsistencies in sample preparation and the varying specimen sizes as well as their geometry and configuration.
Both scanning and transmission electron microscopy (SEM/TEM) analysis has shown the presence of an intermediate layer between 0.5 and 1.5 mm thick (Ngo et al. 1997a, b; Yip et al. 2001). Depending on the type of GIC, the TEM observations ranged from surface interaction zones consisting of nanometer-sized plate-like structures of calcium and phosphate salt precipitates dispersed among denatured smear layer remnants to plate-like structures being present within the inter-fibrillar spaces of intact, banded collagen fibrils. The inclusion of either smear layer remnants or banded collagen fibrils within the surface intermediate layer may be explained by the aggressiveness of different conditioning protocols used to remove the smear layer and demineralizing the underlying intact dentine. This is associated with the concentration of the polyacrylic acid employed as well as the application time that is recommended by each manufacturer. When the dentine was conditioned with 10 % polyacrylic acid for 10 s (a conventional, clinically recommended protocol), the presence of smear layer remnants within the surface intermediate layer indicated the smear layer was not completely removed. Chemical bonding of polyacrylic acid or polyacrylic acid/maleic acid to the residual hydroxyapatite from the smear layer may result in the retention of these polyelectrolytes on the dentine surface instead of being rinsed off (Yoshida et al. 2000). This could help produce the gel-like, glass-free layer that facilitates subsequent chemical exchange between the leached ions from the setting glass-ionomer matrix and the calcium and phosphate ions from the partially demineralized smear layer. Such a surface intermediate layer that incorporates smear layer remnants was often retained on the dentine surface in specimens that exhibited interfacial or mixed interfacial failures (Yoshida et al. 2000). When a more aggressive conditioning protocol of treating the smear layer-covered dentine with 25 % polyacrylic acid for 25 s was employed, the dentine tubule orifices were rendered patent, and this encouraged the formation of micro-mechanical dentine tubule tags. Moreover, the smear layer was removed completely and the underlying dentine demineralized to a depth of about 0.5 mm.
Hosoya and Garcia-Godoy (1998) reported an absence of cement tags or a hybrid layer when using a highly viscous GIC (Ketac-Molar, 3MESPE, St Paul, MN, USA). Rinsing off the conditioner probably resulted in a collagen-rich zone that contained retained polyelectrolytes. Subsequent ion exchange between the setting GIC and the partially demineralized collagen fibrils could have resulted in the formation of a surface intermediate layer where the inter-fibrillar spaces were not infiltrated completely by the polyelectrolytes. This could have accounted for the lower bond strength observed when 25 % polyacrylic acid was used as the conditioner in that study. It is further speculated that the clinical situation may be worsened when conditioned and rinsed dentine is then desiccated by the operator before the application of the GIC, as collapse of the collagen network during air-drying will further limit polyelectrolyte diffusion (Gwinnett 1994). It could be concluded that complete removal of the smear layer with more aggressive conditioning protocols that effectively ‘etch’ into sound dentine does not enhance the dentine-GIC bond strength. The observation of short cement tags that pulled out of the dentine tubules further suggests that they have a limited micro-mechanical contribution to the ultimate retention of GICs.
4.2.4 Caries-Affected Dentine
The MI operative approach to cavitated carious lesion management aims to minimize the excavation of carious dental tissues and instead encourages their preservation, recovery and repair. Dentine caries results from a bacteriogenic demineralizing acid attack from the cariogenic, stagnating biofilm at the tooth surface followed by further enzymatic destruction of the organic, primarily collagenous, matrix in dentine, if the process is unopposed and uninterrupted for a period of time. This ongoing process causes a histo-pathological wave of tissue destruction, divided descriptively into caries-infected and caries-affected dentine zones based on the bacterial load, extent and reparability of the tissue damage sustained (Banerjee and Watson 2015). In the necrotic, caries-infected dentine zone in the heart of the dentine lesion just subjacent to the enamel-dentine junction (EDJ), the mineral and collagenous organic matrices are irreversibly damaged and the bacterial load high. The deeper caries-affected dentine is hypomineralized but with a partially sound collagenous fibrillar structure, which could be repaired and remineralized by the ongoing reparative biological activity of the dentine-pulp complex. The relatively slow progression of the caries process often allows a reparative biochemical reaction which can help restore the mineralized architecture of this zone, especially after having removed the soft, wet, highly infected layer using a minimally invasive operative approach. The interaction between GIC and wet dentine is in the form of an ion exchange where aluminium, fluoride and calcium/strontium leach out of the cement as the glass is dissolved by the polyacid; at the same time, calcium and phosphate ions also move from the underlying dentine as a result of the initial self-etching effect of the acid-base chemical reaction of the setting cement (Watson 1999; Yiu et al. 2004). The release of fluoride and calcium/strontium ions provides GICs with the potential for remineralization of carious tissues (Ngo et al. 2006), where ion exchange could replenish the demineralized tissues’ lost ions, thus tipping the balance in favour of mineral deposition/precipitation.
There is little evidence published about the immediate bond/sealing effectiveness or the long-term durability of the bonded interfaces produced by GIC to caries-affected dentine (Czarnecka et al. 2007; Alves et al. 2013). It is still unknown if the type of GIC has an effect on its clinical performance, as there is little published evidence to date regarding the bond strength of high-viscosity or resin-modified GICs (RMGICs) to caries-affected dentine. Some studies have evaluated bond strength degradation of GIC when bonded to sound dentine (De Munck et al. 2004; Fagundes et al. 2009). Bissoto Calvo et al. (2014) examined the in vitro bond strength of different GICs (a high-viscosity GIC and RM GICs with and without nano-particle fillers) to sound and caries-affected primary dentine immediately and after 2 years storage in vitro. No statistically significant differences in the immediate bond strength values between the tested materials to either sound or caries-affected dentine were reported. After 2 years, only the RMGIC without nano-particles showed stable bond strength values to both primary sound and caries-affected dentine. Previous to this, Marquezan et al. (2010) reported that a resin-modified GIC showed more resistance to degradation at the bonded interface with caries-affected primary dentine after pH- and load-cycling in vitro, compared to an adhesively bonded resin composite. Conventional GIC adheres primarily chemically to dentine, through the interaction of hydroxyapatite and polycarboxylate functional groups. On the other hand, in RMGICs both chemical and micro-mechanical adhesion are involved, which may contribute to the higher immediate and prolonged bond strength values measured.
Although the presence of nano-particles in the formulation of RMGIC potentially reinforces the material’s strength, the interfacial area between the nano-particles and the organic matrix is hydrolytically unstable and may favour water sorption and degradation over time. Additionally, the chemical bond of the nano-particulate RMGIC to dentine may be weaker than that produced by a conventional GIC or a conventional RMGIC. This may be due to the reduction in the polycarboxylate content, as a result of nano-particle inclusion, reducing the available functional groups to interact with hydroxyapatite.
The type of the substrate (sound or caries-affected dentine) did not appear to affect the bond strength of GICs, regardless of type or storage time in several studies (Way et al. 1996; Czarnecka et al. 2007; Marquezan et al. 2010). This can be attributed to the hydrophilic properties of GICs. Also, the type of adhesion is chemical and not purely micro-mechanical. In contrast, Cehreli et al. (2013) observed differences between the bond strength values of GICs to sound and simulated caries-affected dentine after 18 months; unfortunately, the immediate bond strength was not recorded, making it impossible to conclude if any bond strength degradation had occurred. Moreover, the authors used caries-affected dentine created artificially using acetic acid as a demineralizing solution. This method results in complete demineralization (Marquezan et al. 2009), which is different to that of the pH-cycling process of de-and remineralization employed in many other studies. This final point is a critical one: ‘artificial’ data can be produced when using an artificial substrate, and thus clinical extrapolation of such results must be made with considerable caution. The ideal substrate to use is natural caries-affected tissue which needs to be exposed carefully from naturally carious teeth either in situ or in vitro.
4.3 GIC and Remineralization
Remineralization of demineralized carious dentine using various types of GICs has been demonstrated in several laboratory and clinical studies (Creanor et al. 1998; Ngo 2002a, b). Remineralization can be defined as the deposition of mineral in demineralized defects at a molecular level (Arends and ten Bosch 1986). It has been suggested that the mineral deposited should be apatitic in nature and should not be different from the mineral structure of natural, sound enamel and dentine. Ngo et al. (2006) studied the chemical interaction between a highly viscous GIC and demineralized dentine in vivo to determine the level of ion exchange between them. The material they used was Fuji IX GP (GC Corp, Tokyo, Japan), which includes a strontium-containing glass as opposed to the more conventional calcium-based glass in other GICs. They found that a substantial amount of both strontium and fluoride ions crossed the interface into the partially demineralized caries-affected dentine subjacent to the GIC. As the freshly mixed material is placed against the cavity wall, there is a release of ions from the enamel and dentine also leading to the exchange of ions, termed ‘ion exchange adhesion’. It is suggested that the same ion exchange can occur in the presence of the partially demineralized carious dentine (Ngo et al. 2006). The ions released from both the GIC and the tooth structure will combine to buffer the low initial pH until such time that it rises to a level where ion activity ceases. During this period of activity there will be both fluoride and strontium ions available to promote mineral deposition in areas of demineralized dentine where the calcium ion levels are low, with strontium ions substituting them. It was suggested that this occurs through a diffusion process driven partly by the concentration gradient which exists between the GIC and the dentine with respect to these two elements. As both strontium and fluoride are apatite-forming elements, they react with the demineralized dentine. If the process is controlled purely by diffusion then one would expect to see the level of ionic strontium and fluoride to be highest at the GIC-dentine interface and lowest deeper towards the sound dentine. The above clinical findings support the laboratory evidence that glass-ionomer can contribute directly to the remineralization of carious dentine. However, there are two important requirements for this to happen: firstly, the restoration has to provide a total seal against the external environment, and secondly, there must be intimate contact between the glass-ionomer and the partly demineralized dentine.
4.4 Clinical Studies of GIC Use in the MI Management of Deep Caries
The treatment of deep carious lesions approaching a vital pulp presents a significant challenge to the practitioner. The traditional management of carious lesions dictates the removal of all infected and affected dentine to prevent further caries progress and to provide a sound base of dentine to support the overlying definitive restoration. In order to prevent, or at least minimize, the serious complications of complete excavation of carious dentine close to the pulp (the dreaded pulp exposure), the minimally invasive, tooth-preserving operative ‘stepwise’ excavation approach was developed. This involves initially excavating the more superficial soft, wet, necrotic infected dentine, followed by sealing the lesion with calcium hydroxide and a GIC provisional restoration. Some months later the clinician would revisit the lesion and finally remove all or most of the underlying arrested, dry and often darkly stained dentine. The rationale for this is that by this point, any residual bacteria will not have survived, the residual affected dentine will have remineralized and tertiary, reparative dentine will have been deposited. This will make it easier for the dentist to remove any remaining carious tissue without the risk of exposing the vital pulp (Thompson et al. 2008; Banerjee and Watson 2015). As it became increasingly clear that it is the effective peripheral seal of the restoration that is important in preventing the caries process from continuing within an existing cavitated lesion, a fully minimally invasive or ultraconservative approach was developed; this is also referred to as ‘partial/selective caries removal’. In this method, all of the infected dentine is removed, the peripheral enamel and dentine are prepared to optimize adhesion and the cavity is sealed (with or without indirect pulp protection) with the definitive adhesive restoration. The ‘trade-off’ for avoiding pulp exposure, which more often clinically leads to pulp death (Bjørndal et al. 2010), is retaining a layer of potentially radiolucent, affected dentine beneath the definitive restoration (see Fig. 4.2e). This can be defended by citing the substantial evidence that exists in the literature showing that cariogenic bacteria isolated from their source of nutrition by a restoration of sufficient integrity either die or remain quiescent and thus, given a vital pulp, pose no risk to the health of the dentition (Ricketts et al. 2013). Foley et al. (2004) compared the cariostatic effectiveness of alternative restorative materials in both selective and complete removal of carious tissue. They used a split-mouth design in 44 patients (aged 3.7–9.5 years) who had at least one pair of previously unrestored primary molars that had no pulp involvement. One tooth of each pair underwent complete caries removal, and the other had incomplete, selective caries removal followed by restoration using copper phosphate cement, GIC or a material ‘of the operator”s choice’ (such as amalgam). At 24 months post treatment, teeth that had undergone selective caries removal followed by restoration with copper phosphate cement exhibited greater abscess or sinus formation than did teeth that had undergone other treatments. Teeth treated with GIC alone after selective caries removal exhibited a durability and effectiveness comparable with those placed in teeth that had undergone complete caries removal.
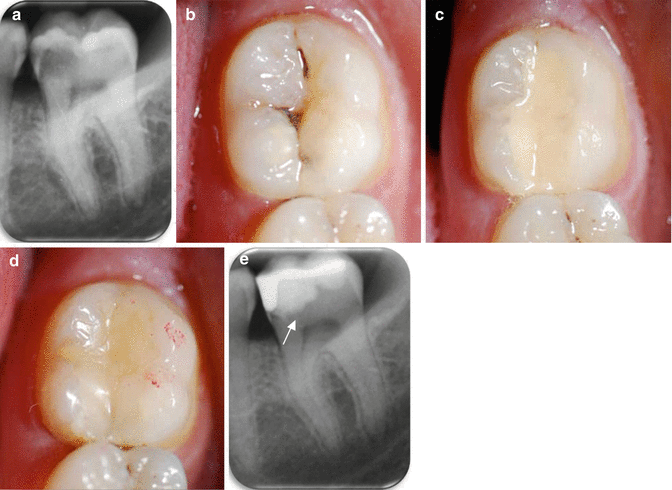

Fig. 4.2
(a) Pre-operative periapical radiograph of LL6 and LL7 showing early caries on the distal aspect of LL6 and a large mesio-occlusal carious radiolucency associated with LL7. The LL7 exhibited symptoms of acute pulpitis and positive pulp sensibility tests and showed no loss of lamina dura/widening of the periodontal ligament space around the root apices in the pre-operative radiograph. (b) An occlusal clinical image of LL7 showing the underlying shadowing of the lesion mesially and cavitation in the midline fissure. (c) After minimally invasive selective caries removal, a GIC was placed as a provisional restoration. (d) At the 1-month review, the GIC was cut back and a resin composite restoration veneered onto its surface as a closed sandwich technique. (N.B: The red marks show the occlusal articulation at the 1-year review stage). (e) The 1-year post-op periapical radiograph: the differences in radiopacities of the GIC and overlying resin composite can be observed. The tooth-restoration complex was sound and the pulp remained vital. The slight radiolucency (arrowed) at the dentine border adjacent to the GIC is the retained caries-affected dentine, and is inactive (Hashem et al. 2015)
Marchi et al. (2006) studied the effectiveness of two materials as indirect protective pulp liners, a setting calcium hydroxide and a RMGIC, in the treatment of 27 primary molars with deep caries. Four years post treatment, the success rate using the former was 88.8 % and using the GIC was 93 %. The investigators defined ‘success’ essentially as the absence of any ‘clinical or radiographic signs or symptoms of irreversible pulp pathologies or necrosis’. The authors concluded that ‘indirect pulp capping in primary teeth arrests the progression of the underlying caries, regardless of the material used as a liner’. In order to provide evidence that the caries process was arrested in the sealed lesions, they sampled teeth for bacterial culture at periods ranging from 1 week to 2 years; at the latter stage, they found a substantial decrease in the number of cultivable micro-organisms in sealed lesions when compared with the unsealed control teeth. Interestingly, they found the greatest amount of bacterial reduction within 2 weeks after treatment. In another microbiological study of dentine samples taken from 40 carious lesions before and after undergoing atraumatic restorative treatment (ART), Bonecker et al. (2003) found significant reductions in the frequency and proportions of the total viable mutans streptococci (but not lactobacilli) in restorations sealed with GIC.
A more recent randomized clinical trial has compared the use of GIC and a calcium silicate cement to restore deep carious cavities in patients (Hashem et al. 2015). The affected teeth were symptomatic with acute pulpitis. Baseline periapical radiographs, CBCT (cone beam CT) and a full clinical examination were carried out before minimally invasive selective caries removal was performed using burs and hand instruments, assisted with Carisolv™ gel. No pulp exposures occurring at this stage of treatment were included in the study. These deep cavities were restored either with a high-viscosity GIC, Fuji IX (GC Corp, Tokyo, Japan), or a setting calcium silicate cement, Biodentine™ (Septodont, Saint-Maur-des-Fossés, France). They were reviewed after 1 month, and assuming the clinical signs and symptoms indicated healing, these provisional restorations were veneered with a resin composite (a layered definitive restoration – see Fig. 4.2). At the 1 year review, it was clear that both materials had a similar 83 % success rate in maintaining tooth structure as well as pulp vitality and the layered restorations were faring well. Thus, on the basis of the evidence cited, it can be reasonably concluded that the removal of all infected dentine in deep carious lesions is not required for successful caries treatment, provided that the restoration can seal the lesion from the oral environment effectively.
4.5 GIC and Atraumatic Restorative Treatment (ART)
A number of countries have already banned or are considering banning the use of dental amalgam, partly in response to the Minamata Treaty agreed by the United Nations Environment Programme (UNEP 2013). Since then, both the International Dental Federation (FDI) and the World Health Organization (WHO) have called for alternatives to amalgam to be developed for use to operatively manage carious lesions. One alternative is to use currently available glass-ionomer cements. Its high-viscosity variant has become the material of choice for atraumatic restorative treatment (ART). This minimally invasive caries management approach, involving the use of hand instruments only and the placement of a high-viscosity glass-ionomer cement (HVGIC), is considered an alternative to the more traditional maximally invasive restorative treatments (Raggio et al. 2013; Holmgren et al. 2013). In terms of restoration survival, a systematic review concluded that ART/HVGIC and amalgam restorations of the same size, type of dentition and follow-up period are equally successful clinically. However, because of the limited number of suitable data sets for the analysis, the authors of the review suggested that further studies should be carried out to confirm these findings (Mickenautsch et al. 2010).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


