Introduction
The major side effect of orthodontic treatment is orthodontically induced inflammatory root resorption. Fluoride was previously shown to reduce the volume of the root resorption craters in rats. However, the effect of fluoride on orthodontically induced inflammatory root resorption in humans has not yet been investigated. The aim of this study was to investigate the effect of high and low amounts of fluoride intake from birth on orthodontically induced inflammatory root resorption under light (25 g) and heavy (225 g) force applications.
Methods
Forty-eight patients who required maxillary premolar extractions as part of their orthodontic treatment were selected from 2 cities in Turkey with high and low fluoride concentrations in the public water of ≥2 and ≤0.05 ppm, respectively. The patients were randomly separated into 4 groups of 12 each: group 1, high fluoride intake and heavy force; group 2, low fluoride intake and heavy force; group 3, high fluoride intake and light force; and group 4, low fluoride intake and light force. Light or heavy buccal tipping orthodontic forces were applied on the maxillary first premolars for 28 days. At day 28, the teeth were extracted, and the samples were analyzed with microcomputed tomography.
Results
Fluoride reduced the volume of root resorption craters in all groups; however, this effect was significantly different with high force application ( P = 0.015). It was also found that light forces caused less root resorption than heavy forces. There was no statistical difference in the amount of root resorption observed on root surfaces (buccal, lingual, mesial, and distal) in all groups. However, the middle third of the roots showed the least root resorption. With high fluoride intake and heavy force application, less root resorption was found in all root surfaces and root thirds.
Conclusions
Fluoride may reduce the volume of root resorption craters. This effect is significant with heavy force applications ( P <0.05). The cervical and apical thirds of the root showed significantly greater root resorption after the application of buccal tipping force for 4 weeks.
Fluoride is the most commonly used chemical agent in preventive dentistry. It has been shown that fluoride reduces caries up to 50% by changing the properties of enamel. Due to its high electro-negative activity, fluoride readily reacts with its surroundings. At the molecular level, fluoride replaces the hydroxyl group of calcium hydroxyapatite crystals and forms calcium fluoroapatite crystals. Fluoride enhances crystal growth, and this newly formed structure is more crystalline, more stable, and less soluble in acid; therefore, it can be considered more resistant to demineralization. At the cellular level, it is believed that fluoride inhibits osteoclastic activity by blocking the calcium ion release that directly suppresses osteoclastic activity. Moreover, it has been shown that fluoride increases the number of osteoblasts and stimulates the formation of new bone.
In a human body, fluoride is almost entirely stored in mineralized tissues. The concentration of fluoride in mineralized tissues can vary according to the level of fluoride intake, the exposure period, and interrelated factors such as the stage of tissue development, vascularity, surface area, porosity, and the degree of mineralization. It was postulated by Zipkin et al and Zipkin and McClure that fluoride is incorporated during bone formation and has a decreased diffusion rate into already formed bones. Several studies have shown that, among the mineralized tissues, the concentration of fluoride was greatest in cementum. The slow rate of cementum deposition can explain this, since rapidly forming tissue would not have sufficient time to accumulate fluoride. Stepnick et al also reported that the concentration of fluoride in cementum increases with age.
Orthodontically induced inflammatory root resorption is an inevitable complication of orthodontic treatment. It has been shown that more mineralized cementum is more resistant to root resorption, and also it has been suggested that the changes on mineral content of cementum might have an influence to avoid orthodontically induced inflammatory root resorption. Foo et al previously investigated the effect of fluoride on root resorption in rats. They found a reduction in the volume of the resorption craters, but this effect was not statistically significant. However, in our previous study, we found that fluoride significantly decreased the volume, depth, and roughness of resorption craters in rats when it is given systemically from birth and also that the longer fluoride was administered, the less root resorption occurred.
To date, to our knowledge, no study has been conducted to investigate the effects of fluoride on orthodontically induced root resorption in humans. Our aim in this study was to quantitatively evaluate whether high and low levels of fluoride in drinking water affect orthodontic root resorption when heavy and light orthodontic forces are applied.
We hypothesized that fluoride might have a great influence to reduce root resorption by increasing the hardness of cementum and, therefore, increase its resistance to clastic activity as well as reduce the clastic cell activity.
Material and methods
The sample consisted of 48 maxillary left first premolars from 48 patients (23 male, 25 female; mean age, 15.27 years; range, 11.75-20 years) who required premolar extractions as part of their fixed appliance orthodontic treatment. Patients were selected from 2 cities in Turkey, having concentrations of ≤0.05 and ≥2 ppm of fluoride in the public water. They were recruited according to strict selection criteria: (1) no previous dental treatment to the teeth to be extracted, (2) no previous trauma to the teeth to be extracted, (3) no previous orthodontic treatment involving the teeth to be extracted, (4) no past or present signs or symptoms of periodontal disease, (5) no past or present signs or symptoms of bruxism, (6) no significant medical history, (7) no physical abnormality concerning the anatomy of the craniofacial or dentoalveolar complex, (8) completed apexification, and (9) residence in the particular region from birth without migration.
Ethics approval was given by the medical faculty ethics committee of the Ondokuz Mayis University in Turkey. All subjects and their guardians consented to participate in this study after receiving verbal and written explanations.
The subjects were randomly divided into 4 groups (12 in each) according to their systemic fluoride intake from public water and the magnitude of force applied on their maxillary left first premolars. Group 1 HH was high fluoride intake and heavy force; group 2 LH was low fluoride intake and heavy force; group 3 HL was high fluoride intake and light force, and group 4 LL was low fluoride intake and light force ( Fig 1 ).
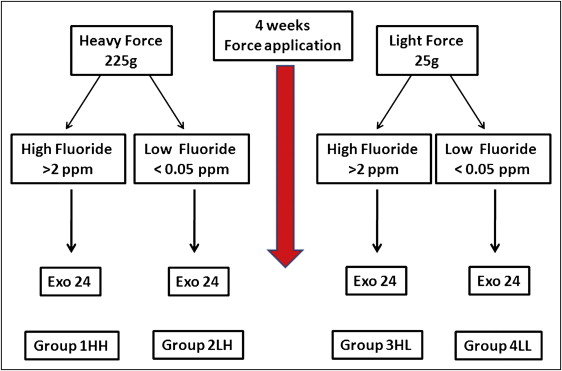
The patients’ maxillary first premolars were exposed to either light forces (25 g; groups 3 HL and 4 LL) or heavy buccal tipping orthodontic forces (225 g; groups 1 and 2) for 4 weeks.
Speed brackets with a 0.022-in slot (Strite Industries, Cambridge, Ontario, Canada) were bonded on the buccal surfaces of the maxillary first permanent molars and first premolars. Self-ligating brackets were selected to allow for standardized ligation of the experimental teeth. In groups 3 and 4, 25 g of buccally directed force was induced by a 0.016-in beta-titanium-molybdenum alloy cantilever spring (Rematitan; Dentaurum, Ispringen, Germany) ( Fig 2 , A and B ). In groups 1 HH and 2 LH, 225 g of buccally directed force was induced by a 0.017 × 0.025-in beta-titanium-molybdenum alloy cantilever spring (Beta III Titanium; 3M Unitek, Monrovia, Calif). The force magnitude was measured with a strain gauge (Dentaurum). Light-cured band cement (Transbond Plus, 3M Unitek) was bonded on the occlusal surfaces of the mandibular permanent first molars to minimize occlusal trauma to the maxillary first premolars during the experiment ( Fig 2 , C ).
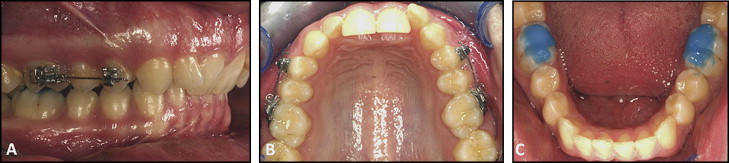
After 28 days of tooth movement, the maxillary left first premolars in all groups were extracted. The samples were sterilized and stored as described previously.
All samples were scanned by a high resolution x-ray microcomputed tomography system (SkyScan 1172; SkyScan, Aartselaar, Belgium) at the Australian Center for Microscopy & Microanalysis in Sydney. All teeth were scanned from the cemetoenamel junction to the root apex at 16.78 μm magnification and resolution of 11.45 μm pixel size. The x-ray tube was operated at 60 kV and 165 μA. The x-ray detector consisted of a 1024 × 1024 pixel 16-bit charge-coupled device camera with fiber-optic coupling to an x-ray scintillator. Scanning was performed with a 360° rotation around the vertical axis and a rotation step of 0.3° and exposure time of 1.770 seconds. Approximately 1200 radiographs were taken from each tooth and saved as 16-bit tagged image file format (TIFF) files.
After image acquisition, the raw data were then processed with specific software (version 1.4.2; Nrecon, Aartsellaar, Belgium). Two-dimensional images were generated as 1024 × 1024 pixel bitmap images having an 8-bit gray scale dynamic range. To identify and isolate the resorption craters, a 3-dimensional reconstruction of the processed data was created by using VG Studio Max software (version 1.2; Volume Graphics, Heidelberg, Germany) ( Fig 3 ). Then, isolated crater images were processed by Convex Hull 2-dimensional software (CHULL2D; University of Sydney, Sydney, Australia) to quantify the volume of the root resorption craters ( Fig 4 ). To evaluate the distribution of the root resorption, the roots were divided into cervical, middle, and apical thirds in the vertical dimension from the cementoenamel junction to the apex and into fourths when viewed axially, indicating the 4 surfaces of the root (buccal, lingual, mesial, and distal).

Statistical analysis
All statistical analyses were undertaken with the Statistical Package for Social Sciences software (version 16, SPSS for Windows; SPSS, Chicago, Ill). Univariate analysis of variance and pairwise comparisons between the groups were performed. The results were depicted by using box plots. Bonferroni adjustments were made for multiple comparisons. The Pearson correlation c was used to evaluate possible correlations between age, sex, and the volume of the root resorption craters. Statistical significance was set at the P ≤0.05 level. All measurements were done by the same blinded researcher (E.K.).
The samples were rescanned after 6 months of the initial measurements. Analysis of variance was used to calculate the mean square error for repeated measurements in each group. The standard error of measurement was derived from the mean square error. These were in 6.6 mm 3 × 10 −3 in group 1 HH; 3.4 mm 3 × 10 -3 in group 2 LH; 6.2 mm 3 × 10 −3 in group 3 HL; and 4.5 mm 3 × 10 −3 in group 4 LL ( Table I ).
| n | Sex (n) | Age (y) | Root resorption (× 10 −3 mm 3 ) | Root surface area (× mm 2 ) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Average | Range | Minimum | Maximum | Mean | SD | SE | Minimum | Maximum | Mean | SD | ||
| Group 1 HH | 12 | 5 | 7 | 14.9 | 11.75-20 | 5.6 | 221.91 | 69.62 | 65.72 | 6.6 | 254.21 | 372.86 | 309.08 | 38.36 |
| Group 2 LH | 12 | 6 | 6 | 15 | 14-17 | 21.98 | 343.04 | 179 | 119.64 | 3.4 | 219.4 | 378.53 | 296.57 | 45.87 |
| Group 3 HL | 12 | 6 | 6 | 15.4 | 13.1-17.8 | 3.01 | 128.42 | 58.57 | 47.53 | 6.2 | 215.08 | 371.7 | 296.64 | 57.15 |
| Group 4 LL | 12 | 6 | 6 | 15.1 | 13.5-16.5 | 10.22 | 222.77 | 72.08 | 77.41 | 4.5 | 232.16 | 461.42 | 331.53 | 75 |
Results
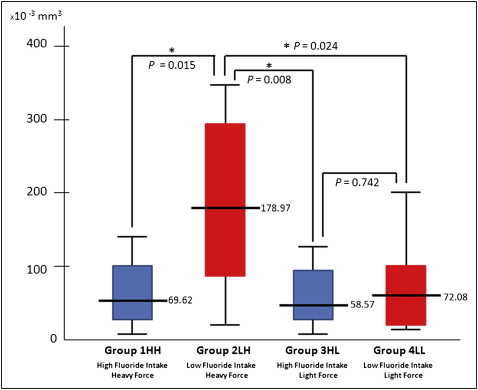
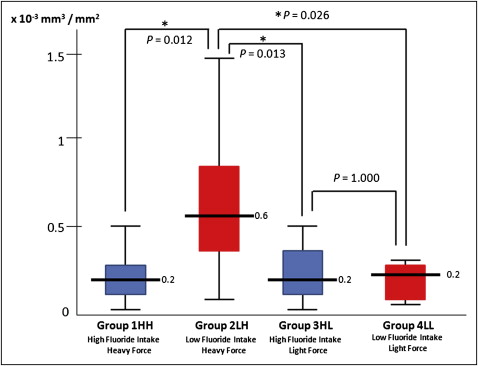
Three-dimensional quantitative analysis of the samples clearly showed that, after 4 weeks of force application, root resorption craters appeared with both the low and high force levels. The mean volumes of the root resorption craters were calculated: 69.62 mm 3 × 10 −3 in group 1 HH; 178.97 mm 3 × 10 −3 in group 2 LH; 58.57 mm 3 × 10 −3 in group 3 HL; and 72.08 mm 3 × 10 −3 in group 4 LL ( Fig 5 , Table I ). It was found that fluoride significantly reduced the volume of the resorption craters under heavy force applications ( P = 0.015). However, this effect was not statistically significant under light forces ( P = 0.742) ( Fig 5 ). Also, it was found that light force application caused significantly less root resorption in patients who drank water with low fluoridation ( P = 0.024) ( Fig 5 ). Although there was a decrease in the average volume of root resorption by light forces in high fluoride groups (groups 1 HH and 2 HL), this effect was not statistically significant. Patients who drank water with high fluoridation (groups 1 HH and 2 HL) had significantly less root resorption than patients who were exposed to low fluoride intake and heavy force application (group 3 LH).
In this study, the root surface area and the volume of root resorption per square millimeter square of root surface were also calculated for each tooth. No significant difference was found between the groups in terms of average root surface area ( Table I ; 309.07 mm 2 in group 1 HH; 296.57 mm 2 in group 2 LH; 296.64 mm 2 in group 3 HL; and 331.52 mm 2 in group 4 LL). The volume of root resorption was distributed per square millimeter of root surface in each sample. The mean volumes of root resorption per square millimeter of root surface area and the comparison between the groups are shown in Figure 6 . Interestingly, when comparing the volumes of root resorption per square millimeter, groups 3 HL and 4 LL become equal ( P = 1.000).

Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


