
8

Pediatric Salivary Gland Disease
The spectrum of diseases of salivary gland origin in children is both different and perhaps less common than that seen in adult populations. However, the anxiety to parents and to pediatricians caused by the presentation of a swelling in the face, oral cavity, or neck will always be high. An attentive, thorough, and thoughtful workup will reassure the family and lead the child safely to the correct series of tests. Basic knowledge of the anatomy, the embryology, and the more common pediatric conditions and their presentations will guide the physician to achieve the correct diagnosis and initiate treatments. The physician must be cognizant that most salivary gland diseases in infants and children are not treated with surgery.
 Embryology and Anatomy
Embryology and Anatomy
The six major salivary glands arise as ectodermal outpouching of the oral mucosa during the sixth to eighth week of gestation. These glandular elements expand into the intercalated ductal structure and eventually into acinar cells.1 The parotid gland is the first and the largest of the major salivary glands to develop. Located just in front of the pinna, the parotid gland extends inferiorly from the zygomatic arch, posteriorly to the pinna and cartilage of the tragus and ear canal, and anteriorly over the ascending ramus of the mandible and on to the body of the masseter muscle. The inferior border of the gland tapers to lie in the groove between the sternocleidomastoid muscle and abuts the inferior edge of the body of the mandible. The parotid gland is tightly sheathed in the parotid-masseteric fascia, causing slight and poorly defined swellings of the cheek when the entire gland is diseased.
The parotid gland also serves as the host for the main trunk and branches of the facial nerve as the nerve exits the skull at the stylomastoid foramen, and travels laterally and anteriorly within the substance of the parotid gland to reach the delicate muscles of facial expression. These branches of the facial nerve create an arbitrary division of the gland when explored surgically and has significance in that the deeper portion of the parotid gland can extend beneath the styloid process and into the parapharyngeal space. Large tumors or masses of the parotid gland may enter the parapharyngeal space and can displace the ipsilateral tonsil fossa into the oropharynx and cause airway symptoms.
The parotid gland is tightly encased between the superficial and deep layers of the investing fascia. The septations within the parotid gland prevent the spread of infection both within and outside the gland, even when suppuration develops in lymph nodes within the parotid gland.2,3
The parotid duct exits from the gland and passes across the masseteric muscle before turning medially through the buccal fat pad to enter the oral cavity as the Stensen’s duct orifice. The duct’s opening is opposite the primary molar dentition in the child under 10 years. In the older child it is adjacent to the second maxillary molar tooth as in the adult.
The submandibular gland is a rubbery, oval shaped mass bound between the anterior and posterior borders of the digastric muscle. The submandibular gland approaches and goes medial to the body of the mandible, again dividing into a larger superficial lobe and a smaller deep lobe. The anterior extent of the gland envelopes the posterior border of the mylohyoid muscle and permits the submandibular duct to turn sharply and medially to reach the floor of the mouth, just lateral to the lingual frenulum. The punctum of this gland is identified by the Wharton’s duct orifice.
The smallest and least clinically significant of the six major salivary glands are the paired sublingual glands that lie just medial and inferior to the punctum of the Wharton’s duct. Approximately 10 small ducts separately enter the floor of the mouth from the sublingual gland. If the ducts are coalesced into a single entity, the duct is renamed the Bartholin’s duct and joins with the Wharton’s duct inferior to the openings on the floor of the mouth.
Saliva
The secretions of the salivary glands provide over 100 to 500 mL of complex fluid to the oral cavity each day. The saliva provides protection and brings moisture to the mucous membranes of the oral cavity, provides sustenance for young tooth buds and deciduous teeth of the alveolar ridges, and prevents the muscles of the tongue from drying and cracking. A dry oral cavity in an infant or young child is one of the earliest and most important signs of dehydration in a young infant, and a frequent portend of death in countries where cholera is endemic to youth and infant populations. Thirst modulates fluid intake effectively when adequate supplies of safe drinking water are available.
Saliva aids in protection from both local and systemic infections by preventing bacteria from entering the mucosal barrier to the head and neck. Secretory immunoglobulins, which are produced in the tonsils and adenoid tissue, migrate into the major salivary glands. The subunits of immunoglobulin A (IgA) then are expressed with a specific antigen-recognizing configuration to combat diseases that may come in contact with the saliva-moistened mucous membranes. Saliva also contains lysozymes, lactoferrin, and minerals to assist in the post-eruption maturation of the primary and secondary teeth. The calcium and phosphate protect against plaque. Erosion of the incisor teeth in infants can commonly occur from sucking too long on the nipple of a sugary solution within a baby bottle.
Hyposalivation
Dry mouth can occur acutely from dehydration, and assessment of the mucous membranes within the oral cavity is an important physical finding. Intervention is best dealt with by immediate oral, nasogastric, or intravenous rehydration. Infusions and bolus delivery of fluid can be quite rapid because young children have a very strong cardiovascular system and can handle moderate fluid overloads. Rarely, intraosseous fluid administration can be an alternative method of fluid intake. Severe dehydration will quickly lessen salivary gland output, which further dries the oral membranes in the mouth. Stasis and thickening of salivary gland secretions predisposes them to infection or abscess formation (e. g., suppurative parotitis).
Complete agenesis of salivary gland tissue in the infant and child is extremely rare and leads to severe xerostomia and loss of teeth.4 Diagnosis is confirmed by magnetic resonance imaging or by radionucleotide studies. Hypoplasia of one salivary gland is rare and not associated with xerostomia.
Chronic conditions that lead to a potential decrease in salivary gland output include diabetes mellitus and hypertension.5,6 Insulin appears to have a direct relationship with salivary gland function in young children.
Radiation therapy for pediatric head and neck malignancies will cause damage to the salivary glands and a subsequent increase in caries within the dentition. Meticulous care of all dental issues and application of dental sealants are both very important prior to commencement of radiation therapy. Squamous cell carcinoma of the nasopharynx, lymphomas, and sarcomatous bone tumors of the mandible, maxilla, or nasal bones are all major risk factors for secondary salivary gland impairment.
Several classes of drugs will induce dry mouth. The perioperative use of the anticholinergic drug atropine is a common example. Postganglionic sympathetic blocking agents have a similar effect. Antidepressant medications that are dibenzoxepine derivatives, including amitryptiline and imipramine, have similar effects on salivary gland secretions. Important mood drugs may need to be discontinued if the side effects are too severe. Titrating medications to reduce drooling may lead to unpleasant dryness of the oral cavity and result in cessation of the medications use and potential benefits.
Intranasal decongestants and antihistamines employed for allergic rhinitis may also cause some dryness of the mouth, but the symptoms are mild and do not result in injury to the oral mucosa. Several medications used to control systemic hypertension may result in chronic mild degrees of oral dryness.
Saliva may change in physical or chemical composition and may go unnoticed if the change is gradual over weeks or months. Patients, particularly children, may not complain. Saliva is generally tasteless, because of a low concentration of glucose and sodium. Active secretion of iodine into the saliva may produce a metallic taste sensation. Cystic fibrosis, which is the most commonly inherited disorder for children, is associated with excessive amounts of salivary calcium and phosphorus. This disorder predisposes to dental calculi and not salivary calculi.7 Diffuse parotid swelling without infection in a child less than 2 years old should prompt a sweat chloride test. Salivary levels of uric acid also increase with gout, which is very rare in children, and can produce salivary gland calculi.8,9 Prader-Willi syndrome (psychomotor and growth retardation, hypotonia, hypogonadism, short stature, and obesity) is associated with a thick saliva.
Overall, chronic xerostomia is quite rare in infants and children.
 History and Physical Examination
History and Physical Examination
The history that leads us to suspect salivary gland disease is defined by symptoms of pain and facial/neck swelling during eating and physical findings that are localized to the major salivary glands or to the oral cavity. Most families bring their child to their family physician or emergency department. Persistent disease will often prompt referral to a specialist.
If the swelling is unilateral, then viral parotitis, obstructions to the ductal system, or adenitis from glandular infections within the parotid is suspected. Bilateral swelling is more commonly associated with systemic diseases, such as sarcoidosis, cystic fibrosis, diabetes mellitus, and starvation. Most of the swelling that is associated with subtotal salivary duct obstruction comes from distention of the ducts and gradually reduces over 2 to 4 hours. The child will refuse to eat because this is painful.
Infections that are sequestered within the lymph nodes that are intimately connected with the submandibular gland or the parotid gland will generally give rise to skin changes of the surrounding tissues. First, there is swelling and erythema. The skin color slowly darkens and becomes red-purple. These changes foreshadow necrosis of the skin and generally lead to suppurative breakdown of the skin with purulent or serous drainage. Inflammatory conditions do not subside quickly, but will subside over 2 to 7 days.
If calculi are the cause, the stone may pass through the duct; this will give immediate and complete relief of symptoms. Unfortunately, this occurs very rarely. Recurrent parotid and submandibular swellings can occur either from stones or from poorly understood inflammatory conditions. Viral parotitis, bacterial sialadenitis, or autoimmune conditions that attack the salivary gland elements must be considered. Exact and reliable clinical tests to confirm these conditions are often lacking.
Physicians are now much more aware of the risk of human immunodeficiency virus (HIV) disease that can be transmitted to infants and young children through maternal exposure, from sexual activity or intravenous drug use. Bilateral enlargement of the salivary glands can occur primarily from HIV, from secondary infection within lymphoepithelial cysts arising within the substance of the parotid gland.
During the physical examination, parents are always protective of their child, and young children tend to be apprehensive. Therefore, careful and gentle inspection is reassuring to both parent and child. Younger children are most comfortable on their parent’s lap. Often, the parent can map out with their index finger the side and site of the swelling. Parents will often be able to estimate the size and describe any associated skin changes of the face and neck. The parents are also very helpful when questioned about skin lesions on the trunk or the extremities because they examine their children regularly with baths and clothing changes.
Palpation should begin only after all information that inspection permits have been obtained. Also, it is important to start with the noninvolved or nontender areas. Gentle, soft, and light fingertip pressure should be applied in a reassuring and nonthreatening fashion. The examiner generally has only one chance to establish reassurance and confidence with the child and the parents. If the child is too fearful to be examined, the examiner may wish to go directly to imaging, which in children is most often accomplished with sedation.
For the older and more mature child, the face and neck are touched, and bimanual palpation is always essential for salivary gland disease. The glands should be carefully palpated for tenderness, areas that are soft and fluctuant, and the mobility of the overlying skin. Granulomatous disease, particularly nontuberculous mycobacterium or sarcoidosis, should be evaluated with skin purified protein derivative (tuberculin; PPD) and chest films. Adenitis of the glands will cause asymmetric swelling.
Bimanual palpation of the salivary gland ducts is critical for complete examination. The ducts are where small calcifications may be detected. These can be only millimeters in size and can slip in and out of the gland itself. Therefore, the gland is massaged from posterior to anterior and from lateral to medial. Small stones, thickened secretions, or blocked puncta can be assessed only when the bimanual palpation is performed in conjunction with careful intraoral visualization of the salivary duct flow and the appearance of the puncta. This part of the examination should be done toward the end because it may evoke some pain and requires very good visualization of the buccal mucosa for the parotid gland and of the floor of mouth for the submandibular and sublingual glands.
Malignant tumors are firm, minimally tender, and can be aggressive and grow quickly. Regional adenopathy should be investigated by palpation. Malignant tumors will often spread outside the gland, invade the fascia, and be fixed to the surrounding muscle, fascia, or bone. The integrity of the surrounding nerves is essential to evaluate. Weakness or asymmetry of the face, lips, tongue, or neck muscles generally means malignant disease. Consultation with pediatric oncology specialists is essential because many pediatric tumors are treated by clinical trials. Malignant tumors in childhood are very rare, but aggressive growth is often seen when these tumors occur.
Photo documentation is also recommended. This can be done with digital cameras and retained in the electronic medical record for reference and consultation use.
 Laboratory Examination
Laboratory Examination
Evaluation of infectious etiologies of salivary gland disease most often involves several laboratory tests. Most often a complete blood count (CBC) with differential diagnosis is obtained. Sedimentation rates and C-reactive protein values are helpful to confirm acute inflammation. HIV testing is useful, particularly if there is bilateral symmetric parotid swelling.
When there is immunodeficiency disease or if there is exposure to a member of the household with tuberculosis (TB), skin tests for TB and/or anergy should be undertaken. Also, multiple views of the chest are very valuable, looking for hilar adenitis.
If there is purulent discharge from Stensen’s duct or Wharton’s duct, a direct culture should be obtained. However, because the cultures are often contaminated with oral flora, the therapy should have strong gram-positive coverage for Staphylococcus aureus and streptococcal species.
Cytological and chemical analysis of saliva and tissue secretions is rarely available or applicable for children. Few pediatric centers see a large case load of malignant disease of salivary gland origin. Therefore, these techniques are not used often enough to maintain and improve skills. Cannulation of the pediatric salivary duct also has been very difficult and is not often performed.
Fine-needle aspiration and cytology, however, have merit, even in children. Cytologic aspirates will require a light, fast general anesthesia. However, when malignancy is suspected and the risks of an open biopsy seem high, this technique will often yield the correct diagnosis when placed in the hands and microscope of an experienced pathologist.
Open biopsies are still preferred when the lesion is demonstrated to be unilateral and confined primarily or exclusively to the lateral lobe of the parotid gland. The submandibular gland can be removed easily, and excisional biopsy should be suggested early if there is a suspicion of malignant disease. Complete removal provides sufficient material and analysis of the tumor borders to better assess prognosis and completion of the excision of the mass. Facial nerve integrity monitors are potentially helpful. Proper surgical technique to identify the trunk of the facial nerve and other anatomical structures adds safety to the surgical experience.
 Radiographic Imaging Techniques
Radiographic Imaging Techniques
Imaging for infants and children involves a variety of techniques (see Chapter 2), from simple plain radiograph films to more sophisticated images, such as computed tomography (CT) scans and magnetic resonance imaging (MRI). Reliable and accurate imaging requires two different elements. First, will the child be awake and immobilized? Second, can sedation be safely and adequately administered to obtain an optimal study? Skilled centers that diagnose and treat high volumes of pediatric patients will most reliably result in a satisfactory study and a happy family and child.
Awake studies include plain radiographs, ultrasounds, and CT or MRI in children 5 years of age or older. Special-needs children, even adolescents, will often require sedation at a site that is in proximity to the imaging department.
Plain films are easy and generally reliable to evaluate the underlying bone structure of the mandible and the skull base. Most plain films are obtained as anteroposterior, lateral, or oblique views. The positioning is important to avoid calcifications that may be hidden by overlying bone structures. Submandibular stones are more often calcified and identifiable (4:1). Dental occlusive views of the floor of mouth are best for submandibular stones. Evaluation of the integrity of the primary and secondary teeth is essential when the area of disease is adjacent to the body of the mandible. Odontogenic abscesses often cause swelling over the mandible or the buccal cheek area.
Sialography is used to image the ductal system, but it is rarely available for children. Placement of catheters within the ducts is very time-consuming, is met with resistance by the child and adolescent, and generally results in high levels of anxiety and frustration. Sialography is optimal for evaluation of ducts for ectasia, strictures, and stones.
High resolution ultrasonography is an effective method to evaluate salivary glands. The parotid gland can be very well visualized. Structures reliably identified include the gland and ductal system, plus the surrounding tissues, including the sternocleidomastoid muscle, mastoid tip, styloid process, carotid sheath, and posterior facial vein.9 Small punctated areas of ductal disease, such as ectasia, can be readily identified as echogenic images through out the gland.10,11
Ultrasound is also favored because this is a rapid tool to evaluate adenitis and to determine whether the node is within the parotid or submandibular gland or extrinsic to it. The node is evaluated for size and whether the center is necrotic and hypoechoic. There is also the loss of central blood vessel flow on color-flow Doppler that confirms the presence of purulent or necrotic material within the mass.
Ultrasound is safe for repeated use in children because of the absence of ionizing radiation. Young parents are also very comfortable with the ultrasound technology because they may have already experienced this technique, which is often performed during pregnancy. Imaging techniques permit accurate measurement of the mass or nodes and allow sequential measurements over days, weeks, or months to assess progression or regression of the lesion.
CT scans with contrast are the diagnostic image of choice for most pediatric-age lesions.12 New and faster CT machines provide completed images and scans within a 5-minute window. Less sedation is required, and many children as young as 4 years of age can cooperate fully. Although there is some radiation exposure, the value of CT images lies in the excellent detail and the ability to alter the contrast of the window to enhance viewing bone, soft tissue, and vascular details.
MRI provides exquisite soft tissue detail and is sensitive enough to demonstrate not only the gland tissue but also the route of the facial nerve within the gland.13 The different spin ratios (T1, T2) permit variations in signal intensity; this leads to recognition of different tumor tissue types. Mixed tumors can be suggested by imaging characteristics. Vascular malformations within the parotid gland, most commonly hemangiomata, are high flow, parenchymatous lesions of intermediate intensity on T1-weighted images and of high intensity on T2-weighted images. MRI techniques are considerably slower than CT. Therefore, children will often need sedation and sometimes general anesthesia for more extensive studies of the torso or head.
 Pathologic Conditions
Pathologic Conditions
Suppurative Parotitis
Bacterial sialadenitis is an acute infection of the major salivary glands that is associated with fever, swelling, and tenderness over the involved gland. The saliva is thickened and purulent, when expressed through bimanual palpation. The salivary punctum will be swollen and erythematous. The infection most often occurs in the very young or weakened infant or child. Purulent parotitis is seen in premature infants, chronically ill children with cerebral palsy or neoplastic diseases, and in poorly nourished children who have undergone major surgical procedures where atropine is administered.
The uncommon neonatal suppurative parotitis is seen mostly in premature babies and male neonates. The higher mucus content and its bacteriostatic protection result in fewer infections of the submandibular gland. Infection can be an oral cavity–derived retrograde ductal infection or from a blood-borne etiology. Fever, anorexia, and failure to thrive are coupled with initially unilateral and subsequent bilateral parotid swelling and tenderness. Parenteral antibiotic coverage for S. aureus and gram-negative bacilli is started until culture results are available. Poor response to medical management is treated with drainage.
S. aureus, S. viridans, S. pneumoniae, and Bacteroides species account for most cases of parotitis in children.14 The parotid gland is more frequently infected, and reports relate this to a higher serous content in the parotid gland saliva when compared with that in the submandibular gland.15 Antibiotic therapy focuses on medications with excellent gram-positive coverage and is combined with very good hydration, as the two main pillars of therapy. Intravenous antibiotics, such as ampicillin/sulbactam, are required for any serious infection with persistent symptoms after 24 hours of oral therapy. Warm compresses, sialagogues, and gentle massage from the gland to the duct’s orifice (e. g., milking the gland) are important.
When the medical therapy is not successful, small abscesses may form. These will often require aspiration or incision and drainage. The surgical procedure depends on localizing the abscess with ultrasound or by CT image techniques and may be best performed in conjunction with an interventional radiologist. Occasionally, the abscess may point near the tragal cartilage or even into the external auditory canal through the fissures of Santorini.16 Dental and periodontal infections are much more common than parotid gland abscess and should be excluded by obtaining dental films in cases where the physical findings are not clear.
Recurrent Parotitis of Childhood
A more common illness is recurrent parotitis that is generally thought to be bacterial and suppurative in nature, but clinical confirmation of this is often absent. What is known is that the condition occurs more often in boys, generally between 3 and 10 years of age. The episodes can reoccur either weekly or monthly, lasting days to weeks. Histological appearance includes dilated intraparenchymal ducts with periductal lymphocytic infiltration.
The treatment and the diagnostic workup tend to remain empiric, and children with persistent disease are often imaged with CT scan. The CT will often show ectasia of the ductal system from chronic inflammation. Serial ultrasounds of the parotid gland are safe and very often useful to identify the presence or absence of intraparenchymal disease.
Empiric therapy with gram-positive coverage is started, and there is often relief of symptoms within 48 hours. Some studies have shown positive cultures of S. pneumoniae, S. aureus, and Haemophilus influenzae. S. viridans has also been reported.17,18 Supportive care is generally all that is needed with the antibiotics. There is speculation about dental trauma, localized strictures of the ducts, or autoimmune etiologies, but few findings occur to confirm these theories.18
Primary management for persistent cases uses serial dilatations of the Stensen’s duct and forcing the child to consume adequate to large amounts of fluids to ensure adequate hydration. Most cases resolve during adolescence, and surgical intervention is not common.
Viral Parotitis
Historically, mumps parotitis constituted the most common cause of parotid enlargement, and in the prevaccination era accounted for its alternate name epidemic parotitis. It was once a common disease of childhood, producing epidemics among children, with a peak age range of 4 to 14 years. It continues to be the most common viral infection of the salivary glands and most frequently affects the parotid gland. Mumps is caused by a paramyxovirus, which is spread by airborne respiratory droplets and is highly contagious. The virus incubates in the upper respiratory tract and parotid gland for a period of 2 to 3 weeks prior to the development of clinical signs and symptoms, and viral shedding can occur for 1 week after the onset of symptoms. Clinically, the patient first experiences constitutional symptoms of fever, malaise, headache (headache is associated with a pleocytosis of the cerebral spinal fluid in 10% of children), neck pain, and myalgias, which are followed by the development of unilateral and then bilateral parotid swelling and pain that is exacerbated by eating. In ~ 30% of children, the salivary gland swelling may be minimal. The other classic manifestation of mumps infection is orchitis, which causes significant discomfort in the acute phase and may be complicated by infertility in the long term. Other potential complications of mumps include encephalitis, pancreatitis, nephritis, and sensorineural hearing loss.
The diagnosis of mumps parotitis is largely clinical, based on the classic presentation of a young patient with bilateral painful parotid swelling (and rarely submandibular swelling) and often in the context of an outbreak of mumps in an unvaccinated population. That said, the diagnosis may be confirmed by serologic testing consisting of hemagglutination antigen testing, mumps A and C antibodies, or complement fixation testing, or by isolating the virus from urine. If serology for paramyxovirus is negative, antibody titers for other viral agents should be obtained. The virus can be isolated from the saliva from 7 days before and up to 9 days after the onset of swelling. The mumps skin test is not helpful as it does not become positive for 3 to 4 weeks after viral exposure. As with many viral syndromes, treatment of mumps is largely supportive. Similar to the treatment of other etiologies of sialadenitis, special attention should be paid to keeping the patient well hydrated, not only to support the patient through the systemic viral infection, but also to assist with good saliva production and flow. Comfort measures such as analgesics and warm compresses may be used. One should note that the swelling of the glands, which may be significant, can take weeks to resolve, but it does typically resolve completely. The patient should be followed for chronic sequelae of the infection, including chronic sialadenitis, infertility, and hearing loss. The vaccine given in combination with measles and rubella vaccines is administered at age 12 months. Viral parotitis now is very rare due to the efficacy of measles, mumps, and rubella (MMR) vaccines. The vaccine is very effective, with a 97% serum conversion rate of over 95%, and the annual incidence of parotitis continues to decline to fewer than 500 cases annually in the United States.19
Human Immunodeficiency Virus
Human immunodeficiency virus infection in children causes a wide spectrum of disease with varied clinical presentation (see Chapter 5). Acquired immunodeficiency syndrome (AIDS) represents the most severe end of the disease process. Parotitis may be present in the earlier stages of the HIV viral infection. Generalized lymphadenopathy often accompanies the mildly symptomatic child, and the presence of cytomegalic viral infection before 1 month of age or oral candidiasis suggests a moderately more aggressive presentation.
Almost one third of children with HIV will have parotid gland enlargement due to lymphocytic infiltration of the gland.20,21 Adenitis within the parotid gland and intraparotid B cell non-Hodgkin’s lymphoma are manifestations of malignant transformation from HIV.22
Cytomegalovirus Infections
Some authors have reported primary postnatal infections of the parotid gland with cytomegalovirus (CMV).23 CMV is highly species specific, and only human strains produce human disease. Horizontal transmission is the result of salivary gland contamination. Excretion rates can be as high as 70% in the 1-to 3-year age group. Some young children can transmit the disease to their parents. Most often the involvement of the major salivary glands is transient and uncomplicated.
Stricture
Narrowing of a major salivary gland duct may result from faulty chewing habits causing trauma to the punctum, calculi within the duct, or external trauma to the duct. Rarely, multiple strictures may occur as the sequelae of a pneumococcal infection involving the duct. The clinical presentation is one of swelling in a single gland during a meal as reflex secretory pressure increases. This swelling may be associated with pain. As the reflex stimulation decreases, the gland slowly empties, and the swelling subsides. Generally, this takes around 2 hours.3
Sialography can be used to diagnose strictures. Dilatation of the duct proximal to the stricture may be seen in long-standing cases (obstructive sialodochiectasis). Strictures may be treated with simple dilation of the duct. Sialodochoplasty is an alternative for refractory cases, with gland excision performed as a last resort.24
Sialolithiasis
Although salivary calculi are relatively common among the general population, they are uncommon in children. The reason for this is not completely understood, although the lower salivary calcium concentration and higher rate of salivary flow in children may protect against calculus formation.25
Sialolithiasis appears to be related to local factors and affects the submandibular gland much more often than the parotid gland.26,27 This is most likely related to the higher calcium concentration, more alkaline pH, and higher mucin content of submandibular saliva. Further, the long, superiorly directed course of Wharton’s duct is more conducive to stasis of secretions than is Stensen’s duct.27
In the pediatric population, boys are affected 3 times as often as girls are. The average age at presentation is 10 years, and many have been symptomatic for a prolonged period of time before the diagnosis is made. Symptoms can range from a slight tenderness in the floor of the mouth to acute sialoadenitis. The most common presenting symptom is submandibular swelling, often associated with pain, which increases with eating and gradually improves afterward.28
The diagnoses can frequently be made on physical examination with bimanual palpation. Radiography should be obtained, even if a stone is palpated, as multiple calculi may be present. Intraoral occlusal radiographs are useful in detecting calculi in the anterior two thirds of the duct, although distal oblique occlusal radiographs are required to evaluate the posterior one third of the duct.28 Alternatively, noncontrast CT scans through the floor of the mouth may be useful in identifying calculi throughout Wharton’s duct and in the gland itself. Sialography or ultrasound may aid in the diagnoses of radiolucent calculi.27
A calculus may occasionally pass through the orifice of Wharton’s duct, either spontaneously or with the use of foods to stimulate salivary flow. If this does not occur, dilation of the duct and sialolithotomy are treatment options. Calculi in the posterior one third of the duct or in the gland itself can be treated with sialadenectomy.27
Cysts
Salivary gland cysts may be acquired or congenital. Acquired salivary cysts develop secondary to trauma or inflammation, or occur as retention cysts. In addition, a neoplasm may undergo cystic degeneration and radiographically mimic a salivary cyst.
Congenital Cysts and Dysgenetic Salivary Glands
Congenital cysts generally involve the parotid gland. These lesions include dermoids, branchial cleft cysts, and branchial pouch cysts. In general, these lesions can be difficult to distinguish from one another, and definitive diagnosis is often deferred until after pathologic analysis.29
Dermoids of the parotid gland may appear as an isolated cystic mass. Dermoid cysts of the floor of the mouth, unlike the ranula, present in the midline. Treatment is by complete surgical excision with facial nerve preservation. Keratinizing squamous epithelium with skin appendages lining the cyst is seen on pathologic analysis of these lesions.
The fetal branchial apparatus is a foregut derivative that develops in the second week of fetal life consisting of four ectodermal clefts, five paired pharyngeal arches, and four endodermal pouches. Less than 5% of branchial anomalies are first branchial cleft malformations. First branchial cleft cysts or fistulas present from the external auditory canal to the angle of the mandible. First branchial cleft cysts have been further classified by Work29 as type I and type II lesions. Type I cysts are duplication anomalies of the membranous external auditory canal (derived from ectoderm) and occur deep within tissue adjacent to the pinna, external auditory canal, and parotid gland. They have a tract to the membranous external auditory canal. Histologically, they are cysts lined by squamous or ciliated columnar epithelium and are associated with lymphoid tissue. They can be histologically indistinguishable from benign lymphoepithelial cysts, and diagnosis is assisted by discovery of a sinus tract. The clinical or histological identification of a sinus tract may confirm branchial cleft origin. The less common type II cysts are duplication anomalies of the external auditory canal and pinna (derived from ectoderm and mesoderm). Often there is an associated sinus tract and stoma opening into the upper neck. They run parallel to the external auditory canal, but they do not have a tract to the membranous external auditory canal. These cysts contain squamous epithelium with skin appendages, as well as cartilage, and can be found either medial or lateral to the facial nerve. Both types of first branchial cleft cysts are associated with repeated infections.
Complete surgical excision with facial nerve preservation, after the acute infection has subsided, is the treatment of choice. In type I first branchial cleft cysts the tract is dissected from the external auditory canal, often using a probe. The excised tract in the external auditory canal can be sutured if small or allowed to granulate and heal by secondary intention. A gauze pack with antibiotic ointment is placed in the external auditory canal. Identification of the facial nerve is essential (Figs. 8–1, 8–2, 8–3).
Branchial pouch cysts are rare lesions that occur in the parotid region, often deep in the retromandibular region. Complete surgical excision with facial nerve preservation is the treatment of choice. Congenital ductal cysts present as parotid swelling in infancy. Generally, no therapy is indicated unless there are repeated infections.30
Another first branchial cleft malformation involves a patent foramen of Huschke. In the third month of fetaldevelopment the anteroinferior surface of the tympanic plate is patent, later closing with fibrous tissue. Persistence of this foramen may lead to otorrhea from external auditory canal fistulas and a parotid swelling from sialadenitis. (More commonly, it involves the temporomandibular joint with pain on chewing.) Otoscopy or endoscopy may reveal a defect in the external auditory canal, confirmed by CT of the temporal bone. In symptomatic patients with involvement of the external auditory canal and the parotid, excision of the fistula and cyst with parotidectomy is necessary to prevent ongoing symptoms.31
FIGURE 8-1 Type I branchial cleft anomaly.
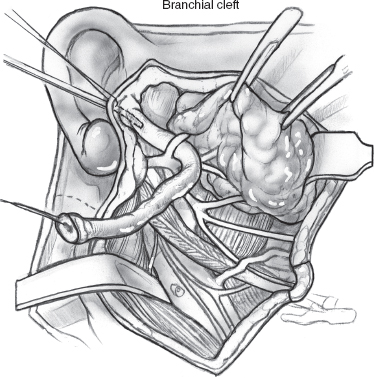
FIGURE 8-2 Excision of the tract requires exposure of the facial nerve.
Preauricular sinuses and cysts are differentiated from branchial abnormalities. They are related to fusion of the ectodermal hillocks from the first and second branchial arches in formation of the auricle. Their location superior to the tragus suggests the tract should not involve the facial nerve. Excision is assisted with the use of a lacrimal probe. The deep plane of dissection is the temporalis fascia. A tiny piece of auricular cartilage is excised where the tract ends. For recurrent preauricular sinuses a wide suprahelical incision down to the temporalis fascia with removal of tissue superficial to this fascia is recommended.
Dysgenetic salivary glands, including the rare congenital polycystic parotid gland, manifests with cysts of varying sizes, with duct differentiation that may include primitive duct buds of the embryonal period or mature intercalated or striated ducts. Duct lumens may contain spheroliths, microliths, or degenerative changes with desquamation. Remnant acini are present between cysts, with no inflammatory cells present. Congenital sialectasia may be unilateral or bilateral and with stagnation of secretions may lead to recurrent sialadenitis. Conversely, duct atresia is rarely reported in submandibular glands. Congenital salivary hyperplasia must be distinguished from sialosis.
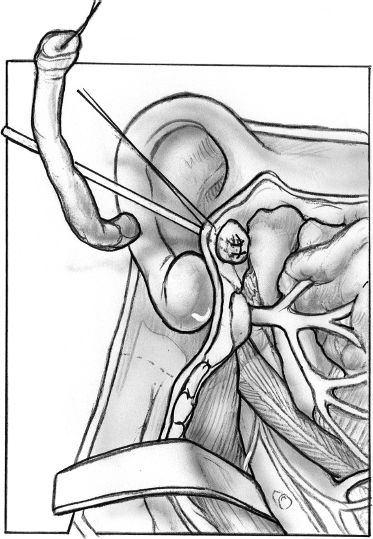
FIGURE 8-3 The external auditory canal defect is sutured.
Acquired Cysts
Mucoceles are pseudocysts that can occur anywhere that minor salivary glands are found, although the lower lip is the most common location reported. The most common presentation is of a painless, fluctuant swelling. A mucocele develops via extravasation of mucin. Pseudocysts form when salivary secretions leak into surrounding tissues usually as a result of trauma to the gland. These secretions cause an inflammatory reaction, which leads to the formation of a wall composed of granulation tissue. Retention cysts occur when there is obstruction of the salivary duct resulting in its expansion and the formation of a true epithelial-lined cyst. Extravasation mucoceles are more common than retention cysts in young people. This distinction is somewhat academic, however, as the treatment is the same for both lesions. Complete surgical excision along with the associated glandular tissue is the treatment of choice.32
Ranula is the term used for a mucocele or retention cyst of the sublingual gland. The term is derived from the Latin word rana (“frog”) because the blue translucent swelling typically seen in the floor of the mouth in these patients resembles the underbelly of a frog.33
A ranula is classified as being either simple or plunging. A simple ranula involves the sublingual space only, whereas a plunging ranula extends posterior to the mylohyoid muscle into the neck. A simple ranula may be either a retention cyst or an extravasation pseudocyst. A plunging ranula is always an extravasation pseudocyst.34
The clinical presentation varies depending on the type of ranula present. The simple ranula will most often present as a bluish, nontender, fluctuant mass on one side of the floor of the mouth. Simple ranulas are mostly asymptomatic, but they can lead to airway obstruction. A soft, painless, ballotable cervical mass is the most common presentation for a plunging ranula. A plunging ranula extends from the floor of the mouth, below the mylohyoid muscle, to the neck. If a cervical cyst is present without intraoral presentation, the diagnosis is more challenging. Plunging ranulas must be differentiated clinically from lymphangioma, dermoid cysts, or hematoma. Imaging, including CT or MRI, may be useful in distinguishing a plunging ranula from a variety of other cystic neck masses.35
Several methods of treatment have been reported, including excision of the ranula, with or without ipsilateral sublingual gland excision, marsupialization, cryosurgery, and observation for spontaneous resolution. The latter will generally occur within 5 months, if at all.33 Recurrence is prevented by excision of the ranula with the sublingual gland; however, one recent study found no increased recurrence rate for simple ranulas treated with marsupialization, compared with those treated with excision of the tumor and associated sublingual gland.34 Complete excision of the lesion, along with removal of the ipsilateral sublingual gland, is the treatment of choice for a plunging ranula.34,35 Surgery in the floor of the mouth must be cautious for the presence of Warthin’s duct and the lingual nerve.
Granulomatous Diseases
Several granulomatous diseases can produce a chronic inflammatory response in the salivary glands. These are uncommon in children, compared with adults. The parotid is the most commonly involved gland. Although diffuse involvement of a gland is sometimes seen, typically patients present with a painless, slow-growing mass within a salivary gland.36,37 Without biopsy, these lesions can be difficult to distinguish from neoplasms. Potential etiologies for granulomatous sialoadenitis include mycobacterial infections, cat scratch disease, sarcoidosis, and actinomycosis.36
Infections of the salivary glands by mycobacteria other than Mycobacterium tuberculosis may present in a fashion similar to that of salivary tuberculosis. In children, over 90% of these infections are secondary to atypical mycobacterial infections. It presents most commonly in children under 5 years of age. There are a multitude of atypical mycobacteria that may produce salivary infection, including Mycobacterium avium, M. aviumintracellulare, M. malmoense, M. scrofulaceum, and M. bovis. The exact route by which these pathogens infect the salivary glands remains unknown; however, possible conduits of infection include the oral cavity, gingival, lips, tonsils, and throat. Infections are most commonly seen in children 16 to 36 months of age, and both the parotid and submandibular glands have been known to be involved.
The classic presentation is one of a painless, unilateral, slow-growing, preauricular or upper cervical mass, unresponsive to antibiotics, which develops a violaceous hue to the overlying skin. Progressive disease may become complicated by sinus tracts. Constitutional signs are noticeably absent. A diagnostic workup similar to that for suspected salivary tuberculosis should be undertaken given the similarity in clinical presentation. The requisite chest radiograph is negative for the cavitary lesions of tuberculosis, and the PPD is nonreactive. CT or MRI will demonstrate a mass with central necrosis. Atypical mycobacterial-specific antigens and polymerase chain reaction techniques for detection of atypical mycobacterial DNA and RNA are evolving. Diagnosis remains largely clinical and is predicated on the exclusion of other salivary mass entities. Fine-needle aspiration biopsy is preferred to incisional biopsy and will help to rule out other causes of salivary swelling; acid-fast staining is unlikely to be diagnostic, and culturing the tissue is difficult and time-consuming, taking as long as 6 weeks to grow. Incisional biopsy or incision and drainage attempts may facilitate the formation of sinus tracts and unsightly scars. Complete excision of the infected gland is considered to be curative, but there is recent evidence in favor of incision and curettage of the diseased gland. Lesser procedures, such as incision and drainage, have a high rate of recurrence.38 Antimicrobial agents have met with limited success. Investigations as to the worth of an antibiotic trial are under way. Drugs under consideration include clarithromycin, ethambutol, rifabutin, azithromycin, and fluoroquinolones in adult populations.
In contrast to atypical mycobacterial infection, mycobacterium tuberculosis infection involving the salivary glands more often has systemic symptoms and a positive PPD. Treatment for such infections includes systemic antituberculosis therapy36,38 (see Chapter 5). Cat scratch disease is covered in Chapter 5.
Sarcoidosis (see Chapter 5) is an idiopathic granulomatous disease, which can involve any of the salivary glands, although the most commonly affected is the parotid gland. Generally, this is manifested by gland enlargement. About 40% of children with sarcoidosis will develop parotid gland enlargement.39 Paralysis of the facial muscles can develop when the disease invades the parotid gland. The paralysis is generally transient and may be associated with uveitis (Heerfordt’s disease). Biopsy of involved glandular tissue may help to establish the diagnosis. Treatment is usually symptomatic with corticosteroids.40
Actinomycosis and Cat Scratch disease are covered in Chapter 5.
Endocrine and Metabolic Disorders
Enlargement of the parotid gland can be seen in up to 10% of patients with diabetes mellitus. This enlargement is due to fatty infiltration of the gland.16 Hypothyroidism can also produce parotid enlargement. In contrast to what is seen in diabetics, parotid enlargement associated with hypothyroidism is a true hypertrophy of glandular tissue. The condition is reversible with correction of the patient’s hypothyroidism. A variety of metabolic disorders, including obesity and malnutrition, are associated with parotid gland enlargement.38 Up to 30% of patients with bulimia may have transient parotid enlargement.41
Neoplasms
Vascular neoplasms are the most common tumors of the salivary gland in children. Some 3.2% of all salivary neoplasms occur in children.42,43 The same tumors that occur in adults occur in children. A solid salivary gland mass in a child is more likely to be a malignancy than in adult.30,42,43 Most neoplasms involve the parotid gland. Tumors in the submandibular or minor salivary glands are more likely to be malignant than in the parotid gland.24,42 In children there is an overall higher female to male sex predilection for salivary gland tumors in general. This is also true for both benign and malignant tumors in children.44
Tissue diagnosis is important in the management of the salivary gland mass. Knowing whether the lesion is benign or malignant allows the surgeon, family, and patient to understand the extent of the planned operation and whether the facial nerve can be preserved.24 If an experienced cytopathologist is available, fine-needle biopsy can serve as a useful guide to differentiate neoplasm from granulomatous disease. Sensitivity for a positive biopsy with a neoplasm is reported to be 92.3%. Specificity for a negative aspiration for tumor has been noted to be 99%.45 CT imaging can be used to increase the diagnostic accuracy when doing a needle biopsy of deep lesions.
Incisional biopsy of the minor salivary gland of the lip is indicated to diagnose Sjögren’s syndrome. Incisional biopsy is rarely indicated for lesions of the major salivary glands, however.44 One should assume that any solid parotid mass in a child is a neoplasm.24 For this reason, once inflammatory disease is excluded, excisional biopsy is preferred to give the pathologist adequate tissue, avoid tumor contamination of the field, and avoid facial nerve injury. It may also be the definitive treatment.3,43 Intraoperative frozen section examination may help with decisions during the course of the procedure.44 Still, radical surgery with facial nerve sacrifice should not be done based on frozen section diagnosis alone.24
CT and MRI both have value in demonstrating whether the lesion is confined to the superficial lobe of the parotid gland. MRI demonstrates better differentiation of tumor from muscle. CT and sialography are important for the diagnosis of inflammatory disease.46 High-resolution ultrasound can also be used to define the relationship of a parotid mass to important adjacent structures.
Benign Tumors
Benign tumors typically occur within the intracapsular portion of the gland.30 The benign mixed tumor (pleomorphic adenoma) is the most common solid salivary neoplasm and the only one that occurs with regularity.9,24,30,42,43,47 any In contrast to vascular lesions, which are most common in infants and young children, they occur in an older age group.30,42 The peak incidence in the pediatric age group is 10 years of age. Benign mixed tumors occur primarily in the parotid gland as a discrete mobile mass. They are usually larger than 1 cm, and they tend to enlarge slowly over time.30 It has been noted that the delay between initial tumor detection and when medical attention is sought can be well over 1 year. Facial nerve involvement is unusual.30 Parotidectomy with facial nerve preservation is the treatment of choice.47 Recurrence is possible.42,44 Long-term malignant transformation in a recurrent lesion has been reported.44
Other types of benign lesions are less common. Basal cell adenoma, papillary cystadenoma lymphomatosum (Warthin’s tumor), xanthoma, neurolemmoma, and lipomas can be seen.30 Neurofibromas are rare but may involve the parotid and submandibular glands in children with neurofibromatosis.42,48
In children less than 2 years of age, the main trunk of the facial nerve and divisions are more superficial and inferior. Beyond 2 years the mastoid tip and tympanic ring form, and the facial nerve takes a deeper position. The marginal mandibularis branch of the facial nerve takes a more superficial position over the mandible compared with adults. Facial paralysis is a significant risk in pediatric parotid surgery.
Malignant Tumors
When one excludes vascular lesions, 50% of all pediatric salivary gland tumors are noted to be malignant.49 The majority of malignant salivary gland neoplasms in children occur within the parotid gland. Pain, rapid growth, cervical adenopathy, and facial palsy are all signs42,49 of a malignant tumor. Malignant lesions tend to occur in older children.49
As in adults, mucoepidermoid carcinoma is the most common (40–50%).30,44 In contrast to adults, however, undifferentiated malignancies are more common in children.42,47 High-grade lesions are aggressive and solitary and tend to grow rapidly. Cystic lesions are generally low grade. Low-grade mucoepidermoid carcinoma generally has a better outcome with appropriate surgery, that is, parotidectomy with facial nerve preservation.43 In high-grade lesions, portions of the nerve may need to be sacrificed.49
Adenoid cystic carcinoma is associated with slow growth, high local recurrence, and distant metastasis, yielding a very poor survival rate.44,49 Pain and facial paralysis are less common.49 Other malignancies include acinic cell carcinoma, rarely adenocarcinoma, and squamous cell carcinoma.49 Lymphoma is rare.3
Wide local surgical excision is indicated for virtually all salivary gland malignancies. Minor salivary gland malignancies are unusual and can be treated with wide local excision.38 Radiation is indicated for facial nerve involvement, aggressive histologic features, adenoid cystic carcinoma with perineural invasion, and cervical lymph node metastasis or residual tumor after38,47 surgery.
Rhabdomyosarcoma is the most common mesenchymal malignancy and rarely occurs in children older than 5 years of age.38 Treatment involves excision and radiation therapy, possibly in conjunction with chemotherapy.38,42,49 Metastatic lesions from areas of the face, scalp, and orbit can occur.3
Vascular Tumors
The vascular lesions include hemangiomas, lymphangiomas including cystic hygroma, and arteriovenous malformations. The most common pediatric parotid mass is hemangioma.16,24,44 This lesion represents 20% of all parotid tumors and 50% of those presenting in the first year of life.30,50,51 Salivary hemangiomas are located in the parotid in 80% of cases. Hemangiomas are solitary in 80% of cases.24 Females are more commonly affected than males.30,38 These lesions can be thought of as hamartomas rather than neoplasms44,52 (Fig. 8–4).
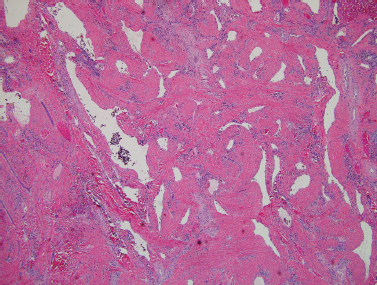
FIGURE 8-4 Hemangioma with medium-size blood vessels present in the subcutaneous tissue (H&E, ×40).
Hemangiomas occur shortly after birth and rapidly enlarge due to cellular proliferation. The acini and ductal structures are unaffected. The parenchyma is replaced by endothelial proliferation. Most are contained within the intracapsular portion of the gland. They can, however, involve skin and surrounding structures with the potential to narrow the ear canal as well as causing significant cosmetic deformity.52 When the lesions involve skin, they are red, rubbery, and lobulated. They are compressible and enlarge with feeding or straining. The lesions will typically involute over time. This usually occurs within the second to fifth year of life, the vascular growth being replaced with fibrofatty tissue.30,38 With this in mind, a conservative, nonsurgical therapy including observation is often the best treatment.38,52 Given the dramatic and occasionally deforming appearance of the child with hemangioma, the family may exert pressure to “do something.”48
Because hemangiomas do tend to involute, accurate diagnosis of this entity is critical to ensure a proper management plan.50 Various imaging techniques can be used, including ultrasound, CT, and MRI.24 As with other lesions, MRI offers the benefit of excellent picture quality, definition of tissue planes, avoidance of ionizing radiation, images in several and planes, and may avoid the need for contrast.46,50
Hemangioma can be associated with complications that require intervention. Hemorrhage, ulceration, infection, platelet sequestration (a localized consumptive coagulopathy is known as Kasabach-Merritt syndrome) and high-output cardiac failure may preclude a conservative plan of observing the lesion.38
Surgery is indicated when these complications occur. Preoperative embolization of feeding vessels can be considered.38 Superficial parotidectomy with facial nerve preservation is the procedure of choice. Complications of surgery such as facial nerve injury, recurrent disease from incomplete resection, and even death can occur. These problems must be balanced against the wait-and-see approach.3 If associated vital structures are at risk, such as the orbit or airway, systemic or intralesional steroids may be indicated. Dosing at 3 to 5 mg/kg/day for several weeks can be initiated.24 Hemangiomas are responsive to steroids in 50% of cases, and the response if effective is often immediate. Interferon use is limited by its toxicity and need for long-term administration.
Lymphangiomas, by contrast, will rarely involute and may enlarge by dilation of existing vessels to encroach on the airway or interfere with feeding. Fifty percent of these lesions are present at birth and 90% by the end of the first year of life.54 Histologically, they appear as cystic dilatations of the lymphatic system30,53 (Fig. 8–5). Lymphangiomas occur most commonly in the parotid gland. They do not replace normal parotid parenchyma. They may arise as part of a cystic hygroma with extensive involvement of the head and neck. These lesions tend to surround nerves and grow beyond tissue planes.
Excision is the standard therapy; however, the extensive nature of the lesion makes nerve injury a high possibility.16 Staged resection of massive lesions of the head and neck may be needed.48 In the hope that the patient may be one of the rare few to experience spontaneous regression, surgery can be deferred until the child is 3 to 5 years of age. Earlier surgery may be needed if the lesion is causing functional problems or getting frequently infected.48 Airway obstruction is more common in lymphangioma of sublingual or submandibular glands. Surgery carries a high chance of recurrence because it is likely that residual cyst will be left.3,16
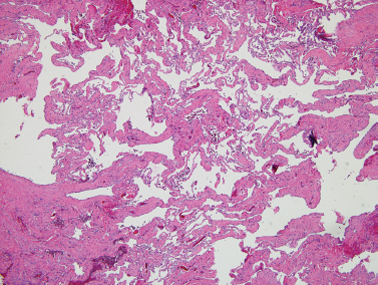
FIGURE 8-5 Lymphangioma with numerous dilated lymphatic vessels present in the subcutaneous tissue(H&E, ×40).
Needle aspiration is discouraged because the fluid will reaccumulate. Furthermore, there is a potential to cause infection or hemorrhage and possibly compromise the airway.3 In the past, attempts to cause inflammation and sclerosis by injection of various materials have been made with limited success. Picinibil (OK-432 Chugai Pharmaceutical Co., Tokyo, Japan) may be the exception.55
Arteriovenous malformations are congenital lesions manifesting early in life. Blood is shunted from arterial vessels directly into the venous system. An arteriogram assists in diagnosing and defining the extent of the problem. Complete surgical resection is preferred.
Sialorrhea (Drooling)
True hypersalivation is a rare condition that is associated with painful oral lesions or heavy metal poisoning with the attendant oral irritation. Hypersalivation is also associated with nausea, Parkinson’s disease, and pregnancy. In teething children, excess oral secretion is common.3
Drooling is the unintentional loss of saliva from the mouth.56 It is a common problem in children with physical or cognitive disabilities.57,58 Although it is normal in children younger than 15 to 18 months of age, in older children it is most commonly associated with neuromuscular disease, particularly cerebral palsy. Poor oral competence and inability to swallow seem to be the primary problem, rather than an excess output of saliva.
Drooling may be associated with perioral skin irritation, dehydration, foul smell, interference with oral intake, soaking of clothing, and damage to electronic communication devices and books. There is a social stigma that potentially may interfere with social interactions and self-esteem. The public views the child as “impaired.”59 There is an increased intensity of caregivers’ time and effort. Aspiration of pooled oral secretions may lead to potentially life-threatening pneumonia.60
Initial assessment should include a determination of how the child holds his or her head, an oral and dental examination, and a full head and neck exam. Adenotonsillar hypertrophy and any condition that causes nasal obstruction, by virtue of leading to mouth breathing, could potentiate drooling.59
Various attempts to measure and quantify drooling and salivary flow have been attempted. Collection of saliva has been tried with various techniques such as using a receptacle, collecting sputum, and measuring volume of secretions absorbed on intraoral cotton rolls.41 Questionnaires to get a subjective idea of the amount of drooling have also been devised.57,59 A basic assessment can be obtained by asking how many bibs or shirt changes occur per day. Seeking a quantitative or qualitative assessment of the impact of drooling for an individual is important in making management recommendations as well as evaluating the outcome of any treatment.
William Crysdale at the Hospital for Sick Children Toronto, Canada advocates a team approach to include a speech pathologist, otolaryngologist, and pediatric dentist. The speech pathologist determines the potential for improved oral motor control. The dentist assesses the health of the teeth and occlusion. The family is involved in decision making.61
Management options include a conservative approach of observation without specific intervention, behavioral modification and working with a speech pathologist, medications to decrease salivary flow, and various surgical procedures.56,58,61 Radiation therapy should be avoided because of the risk of malignant transformation of the gland and the transient response.56,62
Pharmacological Treatment
Treatment with medications is generally aimed at decreasing salivary flow by blocking the cholinergic system’s muscarinic receptors. These drugs have wide-ranging systemic effects. Potential complications of anticholinergics include urinary retention, constipation, blurred vision, drying of bronchial secretions, and restlessness. Atropine has both central and peripheral effects. Hyoscyamine and benztropine have been tried as well.
Glycopyrrolate has several advantages over other medications. It does not cross the blood–brain barrier, so there are no central effects.56 Although it is supplied as an intravenous medication, it can be used orally or via a gastrostomy tube starting at a dose of 1 mg/mL. The dosing can be titrated by modifying the milligram dose or dosing schedule to produce an optimal effect without inducing side effects. If the drug does not produce a full effect, the medication dosage can be increased. If it wears off too quickly, additional dosing can be done.
Scopolamine patches offer the ease of a transdermal route of administration.60 The drug is continually released over a sustained period of 3 days. The difficulty lies in dosing, particularly for small children. The patch should not be cut or trimmed, as the medication will leak, giving unpredictable dosing. There is the risk of the same side effects that can be associated with all of the anticholinergics.
The clinical applications for botulinum toxin (Botox) have increasingly been defined. Botox binds to the presynaptic membrane of cholinergic nerves and produces a chemical denervation of the target tissue.63 Several recent publications have noted a decrease in salivary flow from injection of Botox A in the parotid and submandibular salivary glands.63–65 Patients can be injected under a general anesthesia or after the application of EMLA cream (Astra Zeneca LP, Wilmington, DE). Using ultrasound guidance, the medication can be injected directly into the substance of the gland. Improvement occurs with dosages of 30 U in the parotid glands and 20 to 30 U in the submandibular glands.56,62 Onset of action may begin as soon as 72 hours postinjection but generally occurs anywhere from 7 to 10 days.62,64,65 The amount of response varies.62,63 Duration of response is variable as well, lasting anywhere from 2 weeks to 6 months. Other than pain at the injection site, complications directly related to the drug have not been noted.62,64,65
Surgery
Numerous surgical options have been devised over the years (Table 8-1). Surgical options focus on denervation of the glands, rerouting ducts, duct ligation, submandibular gland excision, or combinations of these procedures.
Bilateral tympanic neurectomy requires a tympanotomy to gain access to the middle ear. The chorda tympani nerve, a branch of the seventh cranial nerve, supplies preganglionic fibers to the submandibular gland. Jacobson’s nerve, a branch of the ninth cranial nerve, acts on the parotid gland. The chorda tympani nerves are sectioned, and a section of mucosa over the promontory along with Jacobson’s nerves is removed. Little long-term improvement has been noted. The risks of tympanic membrane perforation, ossicular injury and hearing loss, and loss of taste generally make this a less than optimal therapy.56,59,66 Tympanic neurectomy has been combined with submandibular gland excision.59
Rerouting the parotid or submandibular ducts directs saliva to a more posterior location in the oral cavity. This technique does not alter salivary volume. The patient with underlying swallowing disabilities may continue to drool. Ranula formation or floor of the mouth edema from submandibular duct rerouting can occur. The rerouted ducts may develop stenosis.58
Wilkie and Brody have reported long-term success in control of drooling. They recommend rerouting of the parotid duct in conjunction with submandibular gland excision.67 Complications include the formation of cysts in the cheek, fistulas with recurrent symptoms, parotitis, and increased dental caries.67 Postoperative technetium scanning has demonstrated persistent submandibular gland function several years after duct rerouting.68
Table 8-1 Surgical Options for Sialorrhea
| Tympanic neurectomy Tympanic neurectomy and submandibular gland resection Rerouting the parotid duct and submandibular duct Rerouting the parotid duct with submandibular gland resection Parotid duct ligation and submandibular gland resection Ligation of the parotid and submandibular ducts |
The gold standard surgical modality to control drooling is bilateral parotid duct ligation (Figs. 8–6, 8–7, 8–8) and submandibular gland excision. This operation focuses on decreasing salivary flow without creating a dry mouth and the attendant discomfort, difficulty eating, and increased risk of caries. In the resting state, the submandibular and sublingual glands produce 70% of saliva. The minor salivary glands produce another 10% of saliva. The parotid gland produces most of the saliva while eating.59,66,69
Removal of the submandibular gland removes a major source of saliva in the resting state. Parotid duct ligation eliminates a major source of saliva with meals.41,59 The remaining sublingual and minor salivary glands continue to provide enough saliva that xerostomia is not generally a problem.69 Ligation of the parotid duct causes gland atrophy. Submandibular gland excision avoids the risk of ranula formation and floor of the mouth edema seen when the duct is rerouted. Success in controlling drooling is ~86 to 88%.59
Surgical risks from parotid duct ligation include sialadenitis of the parotid with painful swelling and possible infection, and fistulization of the parotid duct with recurrent symptoms. Submandibular gland excision risks injury to the marginal mandibular, lingual, and hypoglossal nerves, and bilateral external incisions on the neck. Hospitalization possibly with an ICU stay is recommended for these severely impaired children to monitor their airway.69,70 However, it is usually brief.
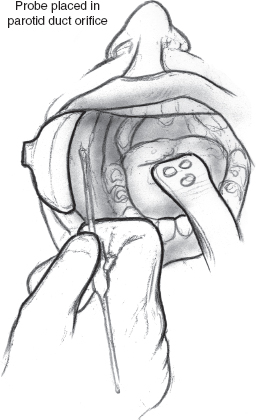
FIGURE 8-6 Lacrimal probe placed in parotid duct orifice.
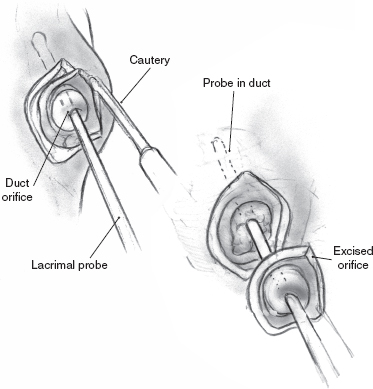
FIGURE 8-7 Excision of duct orifice and surrounding mucosa.
Recently ligation of the submandibular ducts (Figs. 8–9, 8–10, 8–11) in conjunction with parotid duct ligation (four-duct ligation) has been described for chronic salivary aspiration, although its utility for drooling is apparent.70 This procedure takes less time than bilateral parotid duct ligation with submandibular gland excision and avoids the complications associated with gland excision.
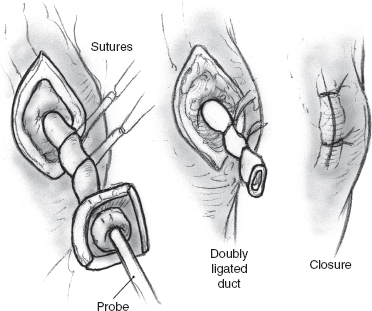
FIGURE 8-8 Duct ligation.
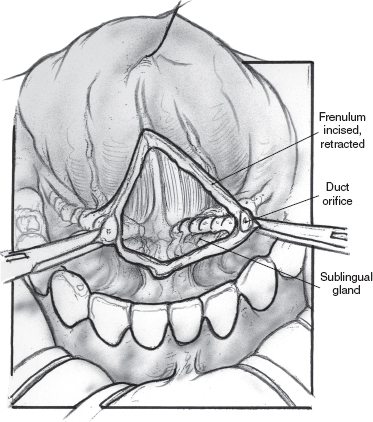
FIGURE 8-9 Midline vertical incision between submandibular ducts (lacrimal probe in duct not shown).
Providing dissection of the submandibular duct is not performed beyond 1 cm of the duct orifice, ranula formation can potentially be avoided. Klem and Mair did cadaver dissections of the floor of the mouth in conjunction with their study.70 They noted that the sublingual glands send multiple ducts that connect with the submandibular duct. These connections occurred beyond 1 cm of the submandibular duct orifice. This finding explains the risk of ranula formation after submandibular duct rerouting.
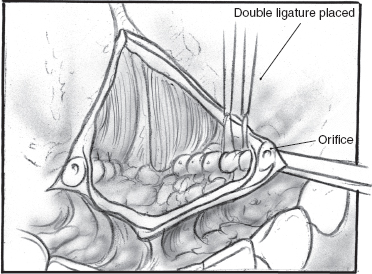
FIGURE 8-10 Double ligation of submandibular duct.
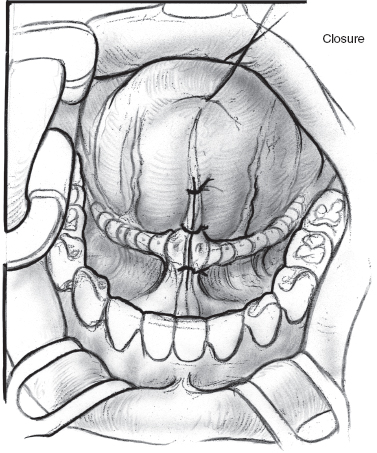
FIGURE 8-11 Loose closure of floor of mouth incision with absorbable suture.
The success rate of four-duct ligation has been estimated at 81%.66 The ease and speed of this procedure may make it a good first alternative to parotid duct ligation and submandibular gland excision.
Ligation of the parotid duct is performed by first retracting the cheek (Figs. 8–6 to 8–8). An injection of a small amount of 1% xylocaine with 1:100,000 epinephrine is made at the duct orifice. The punctum is dilated and cannulated with a lacrimal duct probe. An incision is made around the duct orifice, and the cannulated duct is dissected in the submucosal plane from the surrounding tissues. The duct is doubly ligated with 4.0 silk suture as the lacrimal duct probe is withdrawn. The duct orifice can be cauterized or resected. The mucosa is closed using a 4.0 chromic suture. The procedure is repeated on the opposite side.
Ligation of the submandibular ducts again begins with an injection of 1% xylocaine with 1:100,000 epinephrine (Figs. 8–9 to 8–11). The frenulum between the duct papillae is incised, and the papillae are clamped with a hemostat. The duct is dissected in the submucosal plane for no more than 1 cm. The duct is doubly ligated and the orifice cauterized. The procedure is repeated on the opposite side. The incision can be loosely closed with an absorbable suture or left opened. A single dose of second-generation cephalosporin is administered. Children are sent to the intensive care unit (ICU) postoperatively for airway monitoring.70
Aspiration
Children with significant swallowing dysfunction are at risk for salivary aspiration and subsequent pneumonia.71 This potentially can require frequent hospitalizations and the need for supplemental oxygen. If the child has a tracheotomy, caregivers often spend large amounts of time suctioning the child. This limits the child’s ability to participate in activities, increases caregiver concerns, and negatively impacts on the lifestyle of the child and his or her family.72 Because many children with neuromuscular disease have problems with gastroesophageal reflux, aspirated gastric contents may compound this problem. For this reason pure treatment to control salivary output with medications or surgery may not prevent aspiration. Tracheotomy is not effective in preventing aspiration.72,73 Four-duct ligation and bilateral parotid duct ligation with submandibular gland excision have both been used effectively to control salivary aspiration.70,74
Although several additional surgical procedures have been tried to prevent salivary aspiration, laryngotracheal separation (LTS) appears to be the most effective by most reports.73 The procedure effectively separates the distal airway from the oropharynx. Saliva or refluxed gastric contents cannot enter the lower airway. The procedure is quick, 100% effective in controlling aspiration, with limited complications. LTS can be done in children with or without a preexisting tracheotomy.72,73
The radionuclide salivagram is a simple study to demonstrate salivary aspiration. One cc of technetium 99m sulfur colloid 9 (200 uCi) is placed in the mouth of the child, and the child is scanned from the mouth to the abdomen for 1 hour using a gamma camera. The scan shows whether the radioisotope passes into the stomach or is detected in the trachea and bronchi (Fig. 8–12). If the radionuclide fails to progress from the oral cavity, this is presumptive of significant swallowing dysfunction and a risk for aspiration. The salivagram has a sensitivity of 94% in predicting which children would benefit from LTS. The specificity was 93%.75
Although the procedure is theoretically reversible, it is generally offered to children with devastating neurologic disease. As such, the patient or the family should expect this to be a permanent solution that commits the child to a tracheotomy. The separation of the distal airway from the larynx and oral cavity precludes any verbal communication. Even in the child with severe neurologic impairment, the loss of vocalization has been noted to be a concern to virtually all families prior to the procedure.37 In the rare child who aspirates but can communicate verbally, other alternative approaches to aspiration should be sought prior to LTS.74
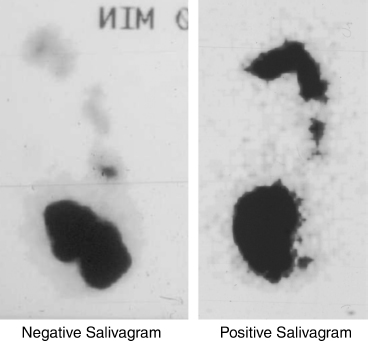
FIGURE 8-12 Salivagram. Image on the left is a negative salivagram showing technetium (Tc 99m) in the stomach 1 hour after instillation of 1 cc in the patient’s mouth. Image on the right is a positive salivagram showing Tc 99m in the mouth with aspiration of material into the trachea and extending into the right and left main bronchi.
The procedure begins by securing the airway with an oral endotracheal tube in the child without a preexisting tracheotomy. If the child has a tracheotomy, the stoma is intubated with an armored endotracheal tube. An apron flap is marked off on the anterior neck at two finger-breadths above the suprasternal notch or just above the tracheotomy tube site. A central semilunar extension is marked to encompass the existing tracheotomy site or, in the virgin neck, to accommodate the future stoma to the distal trachea. The incision is injected with 1% xylocaine with 1:100,000 of epinephrine. The skin is incised, and subplasmal skin flaps are developed superiorly and inferiorly. In the virgin neck the semilunar piece of skin is excised. The peristomal skin and soft tissues of the tracheocutaneous fistula are excised.
The strap muscles are separated in the midline from the thyroid cartilage to the sternal notch (Fig. 8–13). The thyroid isthmus is divided in the midline. The trachea is dissected from all surrounding tissues anteriorly and laterally. A preexisting tracheotomy can create significant soft tissue reaction. The trachea is divided at its anterior and lateral portions at the level of the third to fourth tracheal ring, beveling the incision superiorly. If there is a preexisting tracheotomy, the trachea is transected at the inferior aspect of the tracheotomy stoma. In the virgin neck, the oral endotracheal tube is withdrawn and replaced with a sterile endotracheal tube introduced into the distal trachea and passed under the drapes from the sterile field to the anesthesiologist. The anterior portion of the distal trachea is secured to the lower skin flap with a suture of 0 silk. Before incising the posterior tracheal wall, a small bolus of 1% xylocaine with 1:100,000 of epinephrine is injected into the mucosa. The posterior tracheal mucosa is incised, dividing the trachea in half. The posterior tracheal mucosa is separated from the anterior esophageal wall behind the proximal and distal tracheal segments (Fig. 8–14).
Attention is directed to the proximal tracheal segment. Vertical incisions are made in the midline of the anterior trachea through the second and third tracheal arches (Fig. 8–13). This allows the proximal trachea to collapse upon itself. The proximal trachea is closed as a blind pouch using interrupted stitches of 3.0 nylon or Prolene (Ethicon, Sommerville, NJ) (Fig. 8–14). A superiorly based flap of sternohyoid muscle is created and sutured as a second layer over the proximal tracheal closure with 4.0 Vicryl (Ethicon) as a reinforcing layer (Fig. 8–15).
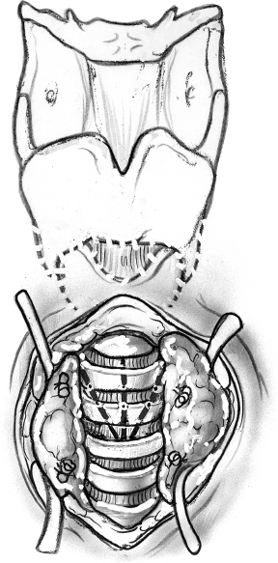
FIGURE 8-13 After division of the thyroid gland, the trachea is exposed, and vertical tracheal incisions are made.
The surgical site is irrigated and meticulous hemostasis achieved. Through a small lateral stab incision, a small Jackson-Pratt drain is placed in the deep aspect of the wound, along the anterior esophageal wall. The distal trachea is sutured to the skin with 3.0 silk, as the incision is closed, maturing the distal trachea as permanent tracheostomy (Fig. 8–16). Antibiotic ointment is applied, and the endotracheal tube is replaced with a standard tracheotomy tube. The child is sent to the ICU. The drain is removed when output is less than 20 cc/24 hours. Gastrostomy tube feeds can be started the day after surgery, but oral intake is deferred until the 7th to 10th postoperative day. The tracheotomy tube can be changed at any time because the mature stoma allows wide access to the distal airway. Good local care prevents peristomal cellulitis. Sutures are removed slowly over several days, a few at time, beginning on the seventh postoperative day.73
Complications are few. Peristomal cellulitis can occur and persist until all sutures are removed. Fistulas are rare and generally managed with local care. Stomal stenosis is possible.73
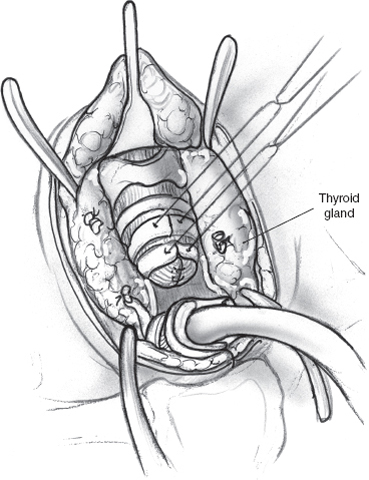
FIGURE 8-14 Closure of proximal trachea.
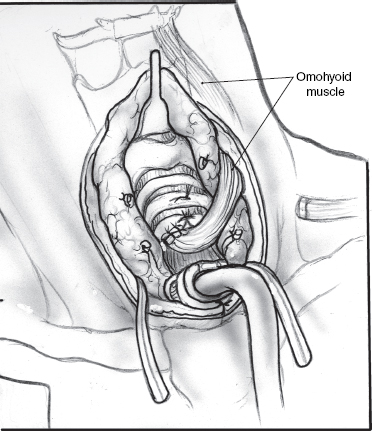
FIGURE 8-15 Strap muscle pedicle flap as a reinforcial layer.
Caretaker satisfaction is high, with fewer reported hospitalizations and pneumonias, and increased ability for the patient to travel. There is also a decrease in home nursing care compared with prior to surgery. Parents generally have concerns prior to the procedure, particularly because of the loss of vocalization, but 100% of families, in one report, noted improved quality of life for both the child and family and would recommend the procedure to others.72
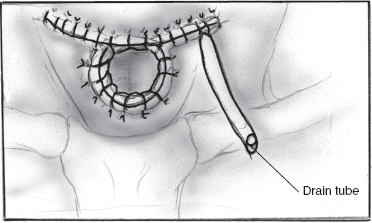
FIGURE 8-16 Closure with suction drain.
REFERENCES
1. Seifert, G Miehlke, A Haubrich, J Chilla, R. Congenital anomalies. In: Seifert, G Miehlke, A Haubrich, J Chilla, R, eds. Diseases of the Salivary Glands. Stuttgart, Germany: George Thieme Verlag; 1986: 63–70
2. Kontis, TC Johns, ME. Anatomy and physiology of the salivary glands. In: Bailey, B, ed. Head and Neck Surgery–Otolaryngology. Philadelphia: Lippincott; 1993: 44–54
3. Seibert, RW. Diseases of the salivary glands. In: Bluestone, CD Stool, SE, eds. Pediatric Otolaryngology. Philadelphia: WB Saunders; 1996: 1093–1107
4. Sucupira, MS Weinreb, JW Camargo, EE Wagner, HN Jr. Salivary gland imaging and radionuclide dacrocytography in agenesis of salivary glands. Arch Otolaryngol 1983; 109 (3): 197–198
5. Liu, FT Lin, HS. Role of insulin in body growth and in the growth of salivary and endocrine glands in rats. J Dent Res 1969; 48 (4): 559–567
6. Henkin, RI Schechter, RJ Hoye, R Mattern, CF. Idiopathic hypogeusia with dysguesia, hyposmia, and dysosmia: a new syndrome. JAMA 1971; 217 (4): 434–440
7. Wotman, S Mercadente, J Mandel, ID Goldman, RS Denning, C. The occurrence of calculus in normal children, children with cystic fibrosis and children with asthma. J Periodontol 1973; 44 (5): 278–280
8. Blatt, IM Denning, RM Zumberge, JH, et al. Studies sialothiasis: the structure and mineralogical composition of salivary calculi. Ann Otol Rhinol Laryngol 1958; 67 (3): 595–617
9. Seibert, RW Seibert, JJ. High resolution ultrasonography of the parotid gland in children. Pediatr Radiol 1986; 16 (5): 374–379
10. Ogata, H Ebihara, S Mukai, K. Salivary gland neoplasm in children. Jpn J Clin Oncol 1994; 24 (2): 88–93
11. Cvetinovic, M Jovic, N Mijatovic, D. Evaluation of ultrasound in the diagnosis of pathologic processes in the parotid gland. J Oral Maxillofac Surg 1991; 49 (2): 147–150
12. Rabinov, K Kell, T Jr Gordon, PH. CT of the salivary glands. Radiol Clin North Am 1984; 22 (1): 145–159
13. Teresi, LM Kolin, E Lufkin, RB Hanafee, WN. MR imaging of the intraparotid facial nerve: normal anatomy and pathology. AJR Am J Roentgenol 1987; 148: 995–1000
14. Myer, CM III. Salivary gland disease in children. In: Gates, GA, ed. Current Therapy in Otolaryngology–Head and Neck Surgery (Vol. 4). Philadelphia: BC Decker; 1990: 174–176
15. Spatt, J. Etiology and therapy for acute pyogenic parotitis. Surg Gynecol Obstet 1961; 112: 391–405
16. Morgan, DW Pearman, K Raafat, F Oates, J Campbell, J. Salivary disease of childhood. Ear Nose Throat J 1989; 68 (2): 155–159
17. Giglio, MS Landaeta, M Pinto, ME. Microbiology of recurrent parotitis. Pediatr Infect Dis J 1997; 16 (4): 386–390
18. Chitre, VV Premchandra, DJ. Recurrent parotitis. Arch Dis Child 1997; 77 (4): 359–363
19. Pickering, LK. Mumps. In: Pickering, LK, ed. Red Book: 2003 Report of the Committee on Infectious Diseases, 26th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2003: 439–442
20. Lee, KC Chung, SW. Evaluation of the neck mass in immunodeficiency virus infected patients. Otolaryngol Clin North Am 1992; 25 (6): 1287–1305
21. Madelena, L Dragan, I Mihordea, M. Clinical and immunological features of the HIV infection associated with chronic hypertrophic parotitis in children. Rom J Virol 1995; 46 (3–4): 135–143
22. Williams, MA. Head and neck findings in pediatric acquired immune deficiency syndrome. Laryngoscope 1987; 97: 713–716
23. Variend, S O’Neill, D Arnold, P. The possible significance of cytomegalic parotitis in infant and early childhood deaths. Arch Pathol Lab Med 1997; 121 (12): 1272–1276
24. Bower, CM Dyleski, RA. Diseases of the salivary glands. In: Bluestone, CD Stool, SE Alper, CM, et al, eds. Pediatric Otolaryngology, 4th ed. Philadelphia: WB Saunders; 2003: 1251–1266
25. Lindman, JP Woolley, AL. Multiple intraparenchymal parotid calculi: a case report and review of the literature. Ear Nose Throat J 2003; 82: 615–617
26. Isacsson, G Lundquist, PG. Salivary calculi an aetiological factor in chronic sialadenitis of the submandibular gland. Clin Otolaryngol 1982; 7: 231–236
27. Bodner, L Fliss, DM. Parotid and submandibular calculi in children. Int J Pediatr Otorhinolaryngol 1995; 31: 35–42
28. Bodner, L Azaz, B. Submandibular sialolithiasis in children. J Oral Maxillofac Surg 1982; 40: 551–554
29. Work, WP. Cysts and congenital lesions of the parotid gland. Otolaryngol Clin North Am 1977; 10: 339–343
30. Myer, C Cotton, RT. Salivary gland disease in children: a review. Part 2: Congenital lesions and neoplastic disease. Clin Pediatr (Phila) 1986; 25: 353–357
31. Sharma, P Dawkins, R. Patent foramen of Huschke and spontaneous salivary fistula. J Laryngol Otol 1984; 98: 83–85
32. Andiran, N Sarikayalar, F Unal, OF Baydar, DE Ozaydin, E. Mucocele of the anterior lingual salivary glands: from extravasation to an alarming mass with a benign course. Int J Pediatr Otorhinolaryngol 2001; 61: 143–147
33. Pandit, RT Park, AH. Management of the pediatric ranula. Otolaryngol Head Neck Surg 2002; 127: 115–118
34. Haberal, I Gocmen, H Samim, E. Surgical management of the pediatric ranula. Int J Pediatr Otorhinolaryngol 2004; 68: 161–163
35. Tavill, MA Wetmore, RF Poje, CP, et al. Plunging ranulas in children. Ann Otol Rhinol Laryngol 1995; 104: 405–408
36. Myer, C Cotton, RT. Salivary gland disease in children: a review. Part 1: Acquired non-neoplastic disease. Clin Pediatr (Phila) 1986; 25: 314–322
37. Shott, SR. Salivary disease in children. In: Cotton, RT Myer, CM III, eds. Practical Pediatric Otolaryngology. Philadelphia: Lippincott-Raven; 1999: 693–710
38. Ibrahim, HZ Handler, SD. Diseases of the salivary glands. In: Wetmore, RF Muntz, HR McGill, TJ, eds. Pediatric Otolaryngology. Stuttgart, Germany: Thieme Medical Publishers; 2000: 647–658
39. Wakley, PE Silverman, JF Holbrook, CT, et al. Fine needle aspiration biopsy as an adjunct in the diagnosis of childhood sarcoidosis. Pediatr Pulmonol 1992; 13: 117–120
40. Rice, DH. Diseases of the salivary glands—non-neoplastic. In Baily, BJ, ed. Head and Neck Surgery–Otolaryngology. Philadelphia: Lippincott; 1993: 475–484
41. Rotteveel, LJC Hongerius, PH van Limbeek, J van den Hoogen, FJA. Salivation in healthy school children. Int J Pediatr Otorhinolaryngol 2004; 68: 767–774
42. Schuller, DE McCabe, BF. Salivary gland neoplasms in children. Otolaryngol Clin North Am 1977; 10 (2): 399–412
43. Camacho, AE Goodman, ML Eavey, RD. Pathologic correlation of the unknown solid parotid mass in children. Otolaryngol Head Neck Surg 1989; 101 (5): 566–571
44. Shikhani, AH Johns, ME. Tumors of the major salivary glands in children. Head Neck Surg 1988; 10: 257–263
45. Frable, MA Frable, WJ. Fine-needle aspiration biopsy of salivary glands. Laryngoscope 1991; 101: 245–249
46. Tabor, EK Curtin, HD. MR of the salivary glands. Radiol Clin North Am 1989; 27 (2): 379–391
47. Orvidas, LJ Kasperbauer, JL, Lewis, JE Olsen, KD Lesnick, TG. Pediatric parotid masses. Arch Otolaryngol Head Neck Surg 2000; 126: 177–183
48. Cook, SP. Salivary gland disorders. In: Mattei, P, ed. Surgical Directives Pediatric Surgery. Philadelphia: Lippincott Williams & Wilkins; 2003: 175–178
49. Baker, SR Malone, B. Salivary gland malignancies in children. Cancer 1985; 55: 1730–1736
50. Huchzermeyer, P Birchall, MA Kendall, B Bailey, CM. Radiology in focus: parotid haemangiomas in childhood, a case for MRI. J Laryngol Otol 1994; 108: 892–895
51. Liu, KK Lam, WW. Parotid hemangioma in infancy: diagnosis with technetium 99m-labeled red blood cell pool imaging. Otolaryngol Head Neck Surg 1995; 112: 780–781
52. Williams, HB. Hemangiomas of the parotid gland in children. Plast Reconstr Surg 1975; 56 (1): 29–34
53. Cook, SP. Salivary gland disorders. In: Mattei, P, ed. Surgical Directives Pediatric Surgery. Philadelphia: Lippincott, Williams & Wilkins; 2003: 175–178
54. Tsui, S-C Huang, J-L. Parotid lymphangioma: a case report. Int J Pediatr Otorhinolaryngol 1996; 34: 273–278
55. Smith, RJH Burke, DK Sato, Y Poust, RI Kimura, K Bauman, NM. OK-432 therapy for lymphangiomas. Arch Otolaryngol Head Neck Surg 1996; 122: 1195–1199
56. Blasco, PA Allaire, JH. Drooling in the developmentally disabled: management practices and recommendations. Dev Med Child Neurol 1992; 34: 849–862
57. Webb, K Reddihough, DS Johnson, H Bennett, CS Byrt, T. Long-term outcome of saliva-control surgery. Dev Med Child Neurol 1995; 37: 755–762
58. Bax, M. Drooling. Dev Med Child Neurol 1992; 34: 847–848
59. Shott, SR Myer, CM 3rd Cotton, RT. Surgical management of sialorrhea. Otolaryngol Head Neck Surg 1989; 101: 47–50
60. Good, WV Crain, LS. Esotropia in a child treated with a scopolamine patch for drooling. Pediatrics 1996; 97: 126–127
61. Crysdale, WS. Management options for the drooling patient. Ear Nose Throat J 1989; 68: 820–830
62. Suskind, DL Tilton, A. Clinical study of botulinum-A toxin in the treatment of sialorrhea in children with cerebral palsy. Laryngoscope 2002; 112: 73–86
63. Shaari, CM Wu, B-L Biller, HF Chuang, S-K Sanders, I. Botulinum toxin decreases salivation from canine submandibular glands. Otolaryngol Head Neck Surg 1998; 118 (4): 452–457
64. Pal, PK Calne, DM Calne, S Tsui, JKC. Botulinum toxin A as a treatment for drooling saliva in PD. Neurology 2000; 54: 244–247
65. Arnold, MG Lindsay, FW Hildebrandt, KH Brewster, DF. Technique for botulinum toxin injection for control of sialorrhea in children. Poster presented at: Annual Meeting of the American Society of Pediatric Otolaryngology; May 2004; Phoenix, AZ
66. Shirley, WP Hill, JS Woolley, AL Wiatrak, BJ. Success and complications of four-duct ligation for sialorrhea. Int J Pediatr Otorhinolaryngol 2003; 67: 1–6
67. Wilkie, TF Brody, GS. The surgical treatment of drooling: a ten-year review. Plast Reconstr Surg 1977; 59 (6): 791–798
68. Hotaling, AJ Madgy, DN Kuhns, LR Filipek, L Belenky, WM. Postoperative technetium scanning in patients with submandibular duct diversion. Arch Otolaryngol Head Neck Surg 1992; 118: 1331–1333
69. Stern, Y Feinmesser, R Collins, M Shott, SR Cotton, RT. Bilateral submandibular gland excision with parotid duct ligation for treatment of sialorrhea in children. Arch Otolaryngol Head Neck Surg 2002; 128: 801–803
70. Klem, C Mair, EA. Four-duct ligation: a simple and effective treatment for chronic aspiration from sialorrhea. Arch Otolaryngol Head Neck Surg 1999; 125: 796–800
71. Waterman, ET Koltai, PJ Downey, JC Cacace, AT. Swallowing disorders in a population of children with cerebral palsy. Int J Pediatr Otorhinolaryngol 1992; 24: 63–71
72. Lawless, ST Cook, S Luft, J Jasani, M Kettrick, R. The use of a laryngotracheal separation procedure in pediatric patients. Laryngoscope 1995; 105: 198–202
73. Cook, SP Lawless, ST Kettrick, R. Patient selection for primary laryngotracheal separation as treatment of chronic aspiration in the impaired child. Int J Pediatr Otorhinolaryngol 1996; 38: 103–113
74. Gerber, ME Gaugler, MD Myer, CM Cotton, RT. Chronic aspiration in children: when are bilateral submandibular gland excision and parotid duct ligation indicated? Arch Otolaryngol Head Neck Surg 1996; 122: 1368–1371
75. Cook, SP Lawless, S Mandell, GA Reilly, JS. The use of the salivagram in the evaluation of severe and chronic aspiration. Int J Pediatr Otorhinolaryngol 1997; 41: 353–361
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


