Introduction
Invisalign plastic aligners (Align Technology, Santa Clara, Calif) are used to correct malocclusions. The aligners wrap around the teeth and are in contact with gingival epithelium during treatment. The purpose of this study was to evaluate the cellular responses of oral epithelium exposed to Invisalign plastic in vitro.
Methods
Oral epithelial cells were exposed to eluate obtained by soaking Invisalign plastic in either saline solution or artificial saliva for 2, 4, and 8 weeks. Cells grown in media containing saline solution or saliva served as controls. Morphologic changes were assessed by light microscopy. The 3-[4, 5-dimethythiazol- 2-yl]-2, 5-diphenyl tetrazolium bromide assay and flow cytometry were used to determine cell viability and membrane integrity, respectively. Cellular adhesion and micromotion of epithelial cells were measured in real time by electrical cell-substrate impedance sensing.
Results
Cells exposed to saline-solution eluate appeared rounded, were lifted from the culture plates, and demonstrated significantly increased metabolic inactivity or cell death ( P <0.05). Saliva eluates did not induce significant changes in cell viability compared with untreated cells. Flow cytometry and electric cell-substrate impedance sensing showed that cells treated with saline-solution eluate exhibited compromised membrane integrity, and reduced cell-to-cell contact and mobility when compared with saliva-eluate treatment.
Conclusions
Exposure to Invisalign plastic caused changes in viability, membrane permeability, and adhesion of epithelial cells in a saline-solution environment. Microleakage and hapten formation secondary to compromised epithelial integrity might lead to isocyanate allergy, which could be systemic or localized to gingiva. However, these results suggest that saliva might offer protection.
It is recognized that unpolymerized monomers can leach out of polymeric materials and possibly cause toxic effects on biologic systems. Potential ranges of cytotoxic effects include an immune reaction to material exposure, cell cycle disturbance, cell apoptosis, and induction of mutagenesis or carcinogenesis. Unfortunately, these effects are not always seen immediately. In orthodontics, aligners are plastic materials used in the correction of malocclusion. Constructed as removable trays that are worn continuously by the patient throughout the day, they wrap around the teeth and contact approximately a third of the gingiva. New aligners are provided every 2 weeks until treatment is complete. Total treatment times range from 6 months to 2 years, depending on the severity of the malocclusion. Because aligners are increasingly used in the correction of most types of malocclusion, treatment time can become protracted for complex cases. With treatment times on the increase, biocompatibility concerns for these polymers are becoming more acute.
In 1998, the Food and Drug Administration approved the Invisalign system (Align Technology, Santa Clara, Calif) for use in patients with permanent teeth and contraindicated its use for malocclusions involving mixed dentition or severe overbite or overjet, and for patients requiring surgical correction, adolescents with a skeletally narrow jaw, and adults with dental prostheses or implants. However, a recent Align Technology report indicated that Invisalign is now approved by the Food and Drug Administration for broader applications, and it is estimated that approximately 1.5 million people use this system worldwide.
Cytotoxic effects of Invisalign have been investigated in human gingival fibroblast and breast adenocarcinoma cell lines. These investigators studied the in-vitro cytotoxic effects of bisphenol A released from Invisalign material and its well-known estrogenic effects. They concluded that Invisalign has no negative effects on the cells tested. The chemical composition of Invisalign plastic does not have the necessary ingredients to release bisphenol A. Invisalign plastic is a polyurethane plastic (material safety data sheet); isocyanate, not bisphenol A, is the component that might pose health issues. Isocyanate usually causes mucous membrane irritation; depending on the duration of exposure, it can cause asthmatic or hypersensitivity reactions. Epithelial cell death, damage to the epithelial layer, or loss of integrity could lead to hexamethylene diisocyanate conjugated protein exposure to the human immune system. As a result, it could create immunologic reactions.
Testing of cytotoxic effects of dental materials by cell culture methods is relatively easy, reproducible, and cost-effective and can be carefully controlled. These tests are more suitable as an alternative to animal experiments, which can introduce uncontrolled variables. In this study, we investigated the oral epithelial cell effects caused by exposure to Invisalign materials. Because gingival mucosal epithelial cells are the first point of contact with this material, they come into close contact for the longest time during treatment. The connective tissue fibroblast, on the other hand, would be exposed only if the epithelium is breached or compromised. We specifically looked into the epithelial cell response through cell death, changes in cell morphology and cell behavior, and integrity of the cell-to-cell barrier function through in-vitro assays. Tight junctions are the apicalmost junctions in epithelial cells and essentially regulate the cell-to-cell barrier function and paracellular permeability across the epithelium.
Material and methods
The human keratinocyte N/ TERT-1 cell line was used in this study. Cells were maintained according to the protocol provided by the James Rheinwald Laboratory, Harvard Skin Disease Research Center (Boston, Mass), where the cell line was purchased. An immortalized cell line was chosen for this study because primary keratinocyte cell lines have limited replicative life spans in culture. Also, it has been reported that this immortalized human keratinocyte cell line initiates the program of terminal differentiation, expresses suprabasal differentiation specific proteins, and forms differentiated epithelia in vitro and in vivo, thereby behaving like normal epithelial cells. Epithelial cells were grown in a monolayer to 75% confluence for the experiments.
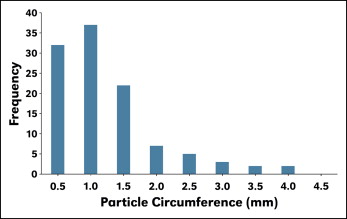
Invisalign plastic aligners were obtained from Align Technology to treat a patient at the College of Dentistry, University of Nebraska Medical Center in Lincoln. Because of a change in the treatment plan, the aligners were not used. Instead, the plastic was powdered with a 12-in half-circle, flat-bottom file, producing particles that were about 86 × 56 μm to 186 × 161 μm in size (length × width), as determined by a measure scope (MM-11B; Nikon, Tokyo, Japan) under 50 times magnification. Particle distribution is shown in Figure 1 . The Invisalign eluate was obtained by soaking 0.1 g of the particulated aligner plastic in either 1.0 mL of normal saline solution or 1.0 mL of artificial saliva for 2, 4, and 8 weeks. The artificial saliva composition conformed to a formula published for the modified Meyer solution.
The cells were grown in media for 24 hours before the experimental conditions were introduced. For the experiments, the saline-solution eluate and the saliva eluate were added in 1:1 or 1:3 dilutions. Dilutions were made with media. To equalize the nutrient availability for growth with dilutions, control cells also received the media diluted with saline solution in the same way as the experimental cells were treated. However, to eliminate the bias with saline-solution based control cells, we did the sensitive electrical cell impedance sensing with cells treated with saliva alone diluted similarly with media in addition to the saline-solution based controls. This experiment demonstrated that the cells in the saliva alone behaved similarly to those in the saliva eluate, but cells in saline solution alone always behaved slightly differently than did cells treated with media only. Therefore, we included saline solution alone in all our experiments as a control to evaluate the effects of treatment.
Keratinocytes were plated in 96 well plates at 3 cell concentrations: 100,000, 10,000, and 1000 cells per milliter, all in triplicate. The cells were incubated for 24 hours in keratinocyte media. The next day, the media were removed, and the cells were washed once with saline solution. Invisalign eluates from 2, 4, and 8 weeks in saline solution or saliva were diluted 1:1 and 1:3 in the keratinocyte media and added to each well. Cells without eluate treatment and wells without cells but with eluate served as 2 experimental controls. All treatment and control experiments were performed in triplicate. Cells were incubated overnight at 37°C with 5% carbon dioxide. The following day, 20 μL of 5 mg per milliliter of 3-[4, 5-dimethythiazol- 2-yl]-2, 5-diphenyl tetrazolium bromide (MTT) was added to each well. The culture plate was incubated for 3.5 hours at 37°C. The media were removed carefully from each well, and 150 μL of MTT solvent was added. The culture plate was covered, and the cells were agitated on an orbital shaker for 15 minutes. Absorbance was read at a wavelength of 570 nm using an absorbance microplate reader (ELx808; BioTek Instruments, Winooski, Vt). The experiments were performed 3 times.
For the flow cytometric determination of membrane integrity, a LIVE/DEAD fixable far red cell stain kit (molecular probes, Invitrogen; Life Technologies, Carlsbad, Calif) was used to assess healthy and compromised cells. Live cells react with fluorescent reactive dye only on their cell surfaces (through cell membrane binding) to yield weakly fluorescent cells. Cells with compromised membranes react with the dye throughout their volume, yielding brightly stained cells. Assays were performed according to the manufacturer’s instructions. Briefly, the cells were grown to 75% confluence in 6 well culture plates. Invisalign eluates (2, 4, and 8 weeks in saline solution or saliva) were diluted to 1:1 and 1:3 in keratinocyte media and added to each well. Cells without Invisalign eluate treatment (control cells) were diluted in a similar manner, with saline solution in the keratinocyte media serving as a control. After 48 hours of incubation, the cells were harvested for a LIVE/DEAD fixable stain assay. The manufacturer’s instructions were followed for performing the assay. Three separate experiments were done.
Epithelial barrier function was measured using an electric cell-substrate impedance sensing system (Applied BioPhysics, Troy, NY). For this experiment, the cells were cultured onto gold electrodes. As the cells grew and became confluent, they constricted current flow and altered impedance. In this study, cell resistance was measured using the electric cell-substrate impedance sensing system continuously for as long as 96 hours. Keratinocytes were grown in 8EW10+ arrays (Applied BioPhysics) that contained 40 circular 250-μm-diameter electrodes and were capable of measuring between 2000 and 4000 cells (confluent monolayers). Each well contained a substrate area of 0.8 cm 2 . Cells (1 ×10 5 ) were seeded into each well, grown to confluence, and treated with the 8-week eluates (saline solution and saliva) for up to 96 hours. Initial tests of the 2- and 4-week eluates in saline solution and saliva did not demonstrate consistent results for MTT and flow cytometric assays. Therefore, only the 8-week saline-solution and saliva eluates were used for the electric cell-substrate impedance sensing system. Cell resistance measurements were based on changes in resistance and capacitance to current flow applied to the electrode arrays at multiple frequencies. A frequency scan was performed to test for the frequency at which the greatest difference in transepithelial resistance values were obtained between the cell-covered and cell-free electrodes. The optimal frequencies to study resistance in keratinocytes appeared to be 250 and 400 Hz. Baseline values were established with culture media alone and compared with electrodes covered with a monolayer of cells. Experimental conditions were run in duplicate on each plate. Three separate experiments were run for each treatment.
Data were analyzed using electric cell-substrate impedance sensing system software, resistance was normalized (subsequent values divided by initial values) and compiled for each condition, and the results were presented as the mean normalized resistance ± standard error of the mean (n = 3). Resistance was normalized by dividing the impedance values from electrodes confluent with cells by the corresponding quantities for the cell-free electrodes using the electric cell-substrate impedance sensing system software. Statistical analysis was performed with a 1-way analysis of variance, and P <0.05 was considered significant.
The electrode arrays (8W1E) and the electric cell-substrate impedance sensing system machine and software were obtained from Applied BioPhysics. Keratinocytes were plated at a density of 105 cells per square centimeter and allowed to spread and attach before impedance was measured. After 24 hours, confluency and viability of the cell monolayer were confirmed by light microscopy. Saline-solution eluate, saline-solution base control, and media control were added to the electrode wells. The electrical impedance of each well was measured every 5 minutes, and up to 16 wells were followed successively. The time series data were normalized. Micromotion measurement was used to monitor time-series impedance fluctuations, which indicated possible cytotoxicity.
Results
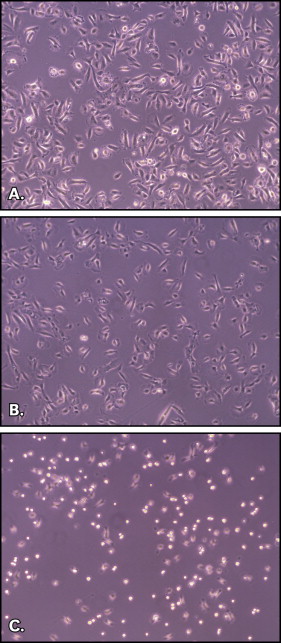
When viewed under a light microscope, keratinocytes treated with saline-solution eluate exhibited rounding and were detached from one another, compared with the untreated cells ( Fig 2 ). Interestingly, cells treated with saliva eluate behaved like the control cells.
Colorimetric MTT assay was used to estimate the number of active and viable cells. The results indicated that cells exposed to 1:1 and 1:3 dilutions of 8-week saline-solution eluate had significantly reduced optical density compared with the control experiments ( P <0.05), indicating either decreased metabolic activity or cell death. Eluates obtained with artificial saliva did not show significant changes compared with the untreated cells ( Table I ).
| Treatment | Dilution | Experiments (n) | Optical density |
|---|---|---|---|
| Saline-solution eluate | 1:3 | 3 | 0.157 (0.06) A |
| 1:1 | 4 | 0.137 (0.05) A | |
| Saline-solution control | 1:3 | 3 | 0.663 (0.06) B |
| 1:1 | 4 | 0.639 (0.05) B | |
| Saliva eluate | 1:3 | 3 | 0.769 (0.06) B |
| 1:1 | 4 | 0.467 (0.05) AB | |
| Saliva control | 1:3 | 3 | 0.575 (0.06) AB |
| 1:1 | 4 | 0.621 (0.05) A |
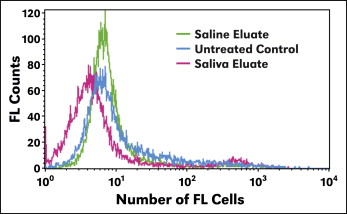
LIVE/DEAD fixable stain assay using flow cytometry measured the fluorescence intensity of cells that were live or compromised. Cells treated with saline-solution eluate showed higher fluorescence signals compared with saliva-eluate treated or untreated cells, indicating that higher fluorescence signals were emitted from the cells. Saliva eluate and cells with no treatment (control) showed similar counts of fluorescence signals ( Fig 3 ). Saliva-eluate treated cells demonstrated similar results as the control cells.