Introduction
Root resorption is an undesirable sequela of orthodontic treatment. It is necessary to establish sensitive methods for identification of teeth at risk for resorption. The x-ray is the traditional method to diagnose root resorption, which is often at a late stage. Some researchers used enzyme-linked immunosorbent immunoassay (ELISA) combined with spectrophotometry to study some biochemical markers of root resorption. However, spectrophotometric detection often has a poor detection limit. Electrochemical detection has inherent advantages over spectrophotometric detection, which is especially suitable for small biologic samples.
Methods
We used ELISA combined with electrochemistry and ELISA combined with spectrophotometry to measure the biochemical marker dentine sialophosphoprotein in gingival crevicular fluid of orthodontic patients (treated for 8-12 months).
Results
Standard dentine sialophosphoprotein was used to calculate the linear regression equation. No significant difference was found between the electrochemical outcome and the spectrophotometric outcome. But the electrochemical results extended the lower end of detection from 5 pg per milliliter (by spectrophotometry) to 0.5 pg per milliliter.
Conclusions
These results showed that ELISA combined with electrochemistry is a reliable and sensitive method to detect dentine sialophosphoprotein in gingival crevicular fluid.
External apical root resorption is an overresorption of cement for unknown reasons that can cause loose teeth or even loss of teeth. It is a common sequela of orthodontic treatment and remains unexplained. According to various studies, about 20% to 100% of patients who receive orthodontic treatment develop root resorption, most of which was mild to moderate, but some was severe. Severe root resorption is a considerable risk factor for the integrity of the dentition in the long run. Among all teeth, the incisors are most susceptible to resorption because of their root characteristics, which concentrate greater stress on the apexes in orthodontic procedures.
Currently, clinical diagnosis for root resorption is often based on radiographic examination, but it brings radiation exposure, which could limit the longitudinal and systematic study of the roots. Moreover, imaging cannot detect root resorption until 60% to 70% of the mineralized tissue is lost; this makes it impossible to monitor root resorption and stop its progress and can result in loss of teeth. Therefore, it is necessary to establish sensitive methods to identify at early stages the teeth with a risk of resorption.
Dentine sialophosphoprotein (DSPP) is a specific protein that is released into the periodontal ligament space during active external root resorption. Mah and Prasad used biochemical assays to detect dentine proteins and found that dentine phosphoproteins are associated with root resorption. Researchers confirmed that dentine phosphophoryn and dentine sialoprotein in the gingival crevicular fluid (GCF) of people undergoing orthodontic treatment can be regarded as biologic markers for monitoring root resorption. Dentine sialoprotein and dentine phosphoproteins are N- and C-terminal proteolytic cleavage products of DSPP, respectively, and belong to the small integrin-binding ligand N-linked glycoprotein family of proteins. Hence, DSPP can be regarded as a marker for the detection of root resorption and monitoring its progress.
Enzyme-linked immunosorbent immunoassay (ELISA) is a traditional method used in biochemical diagnosis and clinical practice. It is easy to use and suitable for most applications. But spectrophotometry, the last step to measure the product in ELISA, often has a poor detection limit. In recent years, ELISA combined with electrochemical detection has been proposed and widely used for the detection of tumor markers or plant viruses. It has been shown that electrochemical detection is inherently advantageous over spectrophotometry with its lower detection limit, wider dynamic range, and consequently higher sensitivity. This was further proved in some studies with small biologic samples. Obviously, studies with GCF could be limited by its small amount. However, previous studies on DSPP in GCF used ELISA with spectrophotometry, and it was hard to quantify the product. We therefore aimed to determine whether electrochemical ELISA is effective in the identification and quantification of DSPP in active orthodontic patients.
Material and methods
Twenty patients were enrolled at the Department of Orthodontics of the dental school affiliated with Capital Medical University in Beijing, China. They were orthodontic patients receiving treatment for 8 to 12 months, including 12 female and 8 male subjects (age range, 13-24 years). Inclusion criteria were good oral hygiene, no periodontal disease, no bleeding on probing, no caries, and no systemic diseases. The type of orthodontic displacement of the incisors was not considered in the inclusion criteria because it was not supposed to affect the comparison of the 2 detection methods.
This project was approved by the institutional review board of Beijing Stomatological Hospital, and informed consents were obtained from the patients.
For each patient, 2 teeth were chosen for GCF collection: the left and right maxillary central incisors. The teeth were gently washed with water, dried, and isolated with cotton rolls to prevent saliva contamination. GCF was collected from the mesial and distal sides of each tooth with a filter paper strip (Tianjin Zhongjin Biological Technology, Tianjin, China), which was inserted 1 to 2 mm into the gingival sulcus for 30 seconds. The same procedure was repeated at a 1-minute interval. As a result, 160 strips of samples were obtained, 2 for each position. After removal, each strip was immediately sealed in a microcentrifuge tube with phosphate-buffered saline solution containing 0.1 m mol/L of phenylmethylsulphonyl fluoride. To retrieve the sample from the paper strip, the GCF was eluted by centrifugal filtration at 15,000 g for 5 minutes with 100 μL aliquots of buffer. Two GCF samples from the same position were then pooled to give a total volume of 200 μL and stored at −70°C. Therefore, 80 GCF samples were prepared, and each was assessed with both electrochemical and spectrophotometric ELISA.
The sandwich ELISA procedure was used for the detection of DSPP in the standard solution of all GCF samples. In general, 50 μL of the DSPP sample was mixed with biotin labeled DSPP antibody and added into the microplate, which was precoated with DSPP monoclonal antibody. Then 50 μL of streptavidin labeled horseradish peroxidase solution was added into each well. After incubating at 37°C for 60 minutes, the wells of the microplate were washed 5 times with 0.02 mol/L of phosphate-buffered saline solution containing 0.05% Tween-20. After washing, 200 μL of substrate solution was added into each well and incubated at 37°C for 30 minutes. Then 50 μL of Britton-Robinson buffer (0.2 mol/L, pH 5.0) was added to each well.
For the electrochemical analysis of the samples, a miniature 3-electrode system was directly inserted into the well, and the second-order derivative linear sweep voltammetric reduction peak was measured with a JP-303 voltammetric analyzer (Chengdu Apparatus, Chengdu, China). The experimental procedure is shown in Figure 1 .
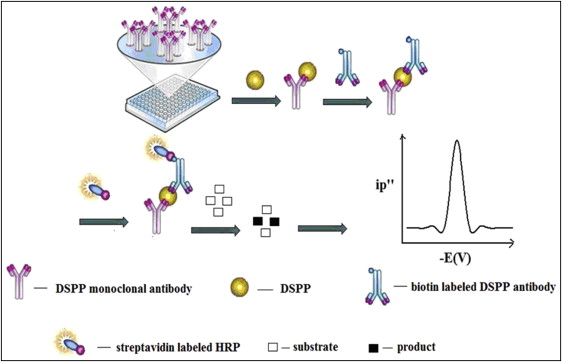
Using horseradish peroxidase as the labeled enzyme, o-tilidine was used as the enzymatic reaction substrate in this research; it can be oxidized by hydrogen peroxide to give an electroactive azo product. Under the optimal conditions, the proposed o-tilidine–hydrogen peroxide–horseradish peroxidase system was combined with double-antibody sandwich ELISA and further used to detect DSPP in GCF. In the electrochemical ELISA procedure described above, the more DSPP in the solution of GCF, the more the labeled horseradish peroxidase was coupled on the microplate and more of the enzymatic product could be formed; this gave a higher reduction peak current.
The prepared GCF solution of each sample was also analyzed with the traditional spectrophotometric ELISA procedure.
In the spectrophotometric ELISA procedure, the more DSPP in the solution of GCF, the more the labeled horseradish peroxidase was coupled on the microplate and more of the enzymatic product could be formed; this gave a darker color.
Statistical analysis
The linear regression equation of DSPP was calculated with standard DSPP solution (Shanghai Jingtian Biological Technology, Shanghai, China), with reference to the range of DSPP shown in previous studies, to further establish the electrochemical method for the detection of concentrations of DSPP. Then the DSPP concentrations in the GCF samples measured by the electrochemical ELISA procedure were compared with those by the spectroscopic ELISA procedure. The analysis was performed with a paired-samples rank sum test, and the level of significance was set at P = 0.05.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


