Fig. 3.1
Anatomy (seven subsites) of the oral cavity (Created by Daniel J. Liebertz)
3.1.2 Types of Oral Cancer
The predominant histopathological classification of oral cavity cancer is squamous cell carcinoma (SCC), which accounts for 84–86 % of new cases [1, 2]. In the most frequently involved subsites – the oral tongue and FOM – rates exceed 94 %. As such, this chapter will be limited to the discussion of SCC. Less common entities include minor salivary gland malignancies (adenocarcinoma, adenoid cystic carcinoma, mucoepidermoid carcinoma, acinic cell carcinoma), verrucous carcinoma, lymphomas, soft tissue and osseous sarcomas, and melanomas. These possibilities should be considered in the differential diagnosis. Tumor behavior, diagnosis, and surgical management of non-SCC malignancies can differ significantly from that of SCC. The reader is encouraged to refer to other resources for further discussion of non-SCC malignancies.
3.1.3 Epidemiology
The oral cavity and lip are the second most common sites of head and neck cancer, behind the larynx [1]. The American Cancer Society estimated that 28,030 individuals will be diagnosed with oral cavity cancer and 5,850 will die from the disease in the USA in 2014 [2]. Cancer of the oral cavity, lip, or oropharynx is the eighth most common cancer in males. Twice as many men are diagnosed as women and the average age at diagnosis is 62 years. The US incidence of oral cavity cancer is slightly declining, in contrast to oropharyngeal cancer, which is on the rise in white men and women in association with rising HPV prevalence [3]. Worldwide, over 300,000 individuals were diagnosed with oral cavity and lip cancer and over 145,000 died from the disease in 2012 [4].
Incidence rates are highest in Whites, followed by Blacks, American Indians/Alaskan natives, Asian/Pacific Islanders, and Hispanics [2]. OC rates in Blacks have declined significantly since 1985; however, this has been offset by a gradual increase in Whites and Hispanics in recent years. The oral tongue remains the most frequently involved subsite, followed by the FOM and alveolar ridge [2]. Between 2002 and 2011, incidence rates rose slightly for the oral tongue, but decreased for the FOM and alveolar ridge. While the lip is considered a subsite of the oral cavity, it is often categorized and managed separately.
3.1.4 Risk Factors
Oral cavity SCC (OCSCC) is a multifactorial disease. Risk factors may be extrinsic or intrinsic in nature. Infectious etiologies – HPV in particular – have become increasingly prevalent, although this trend is clearer in oropharyngeal SCC. OCSCC may arise from some preexisting lesions.
3.1.4.1 Extrinsic Factors
The strong causal link between tobacco use and OCSCC is well established. The carcinogenic effect of tobacco is due to its combination of nitrosamines, tars, and nicotine, which induce cell damage either directly or in a metabolite form. All forms of smoked tobacco, including cigarettes and cigars, are implicated. Multivariate analyses of risk factors for cancers of the oral cavity and oropharynx demonstrated that those who smoke six or more cigarettes per day had an odds ratio (OR) of 4.1 compared with minimal smokers, rising steadily to a maximum of 5.6 for those who smoked 26–35 cigarettes per day [5]. Longer duration and increased cumulative use of cigarettes were positively correlated as well. Former smokers who quit more than 10 years prior had a 50 % reduction in cancer risk compared to current smokers, suggesting a protective effect of smoking cessation.
While smoking is the most popular form of tobacco use in the West, variations of smokeless tobacco are used extensively throughout the world. This includes ground tobacco leaves (snuff) and chewing tobacco [6]. Carcinogenic compounds are directly absorbed through the oral mucous membrane leading to localized cellular damage and inflammatory changes. The risk of OCSCC in smokeless tobacco users is similar to that in tobacco smokers [7]. It is estimated that up to 90 % of smokeless tobacco use is in South Asia. It is not a coincidence that oral cavity cancer is one of the leading causes of death in many of these countries. Alcohol consumption has been implicated as another strong risk factor. Heavy drinkers have up to an eightfold risk of OCSCC compared to minimal drinkers. The type of alcoholic beverage may play a role, as mixed drinkers are at higher risk compared to beer or whiskey drinkers [5]. A significant proportion of the population that develops oral cavity cancer consumes both tobacco and alcohol. The combined effect is multiplicative, rising to a maximum OR of 98.4 in heavy users of both. A similar synergistic effect of tobacco and alcohol has been identified in all cancers of the upper aerodigestive tract.
Betel quid is a chewed preparation of betel leaf and areca nut, which is sometimes mixed with tobacco. It is extremely popular in India, Pakistan, and other Southeast Asian countries. Betel quid chewing has been recognized as the leading cause of oral cavity cancer in this part of the world [8]. It is believed that local mucosal inflammatory changes induced by betel quid progress to oral mucous fibrosis, which has a rate of malignant transformation as high as 19 % [9]. The mixing of betel quid with tobacco may pose an even greater risk than either substance alone [7].
Ultraviolet radiation (UVR) is strongly associated with skin cancers, which include malignant melanoma, basal cell carcinoma, and SCC. The main forms of UVR present in sunlight, and which were shown to be carcinogenic in animal studies, are UV-A and UV-B. A clear trend of increased nonmelanoma skin cancer in regions that receive more UVR based on geographical location has been found [10]. The risk of developing lip cancer is strongly related to lifetime solar radiation (OR = 13.5) and decreased sunscreen use [11].
Viruses are believed to cause 15–20 % of all cancers worldwide [12]. In head and neck cancers, Epstein-Barr virus (EBV) and human papillomavirus (HPV) are the main contributors. Since the discovery of its causal role in cervical cancer, HPV has been further implicated in SCC of the oral cavity, oropharynx, and hypopharynx as well as benign papillomas throughout the upper aerodigestive tract [13]. The rising number of oropharyngeal cancer diagnoses in the USA is attributed to increasing HPV prevalence, particularly of high-risk subtypes 16 and 18 [14]. HPV contains E6/E7 oncoproteins that inactivate important host tumor suppressor genes (i.e., p53 and Rb) and modify the cell cycle to enhance proliferation. Compared to traditional patients with head and neck SCC (HNSCC), HPV-associated disease has a tendency to affect younger populations. Caucasians, males, nonsmokers, nondrinkers, and those with a higher number of sexual partners are at greater risk [15]. HPV-associated HNSCC is increasingly seen as a clinically distinct entity from HPV-negative SCC and has a unique pathogenesis, prognosis, and management paradigm. It is too early to quantitate the impact of multivalent HPV vaccines, which cover both high-risk and low-risk subtypes on HNSCC.
3.1.4.2 Intrinsic Factors
Advanced age is strongly correlated with a number of malignancies, including OCSCC. The incidence is sixfold greater in those over the age of 65 years compared to those under 65, and the risk continues to rise well into the 80s [2]. The rising trend of HPV-associated HNSCC in younger patients may be lowering the average age of diagnosis. As such, malignancy should remain within the differential diagnosis in any patient with suspicious exam findings and an appropriate history.
The prevalence of OCSCC is two- to fourfold higher in males compared to age-matched females. It is unclear if this remains true independent of lifestyle choices such as tobacco and alcohol use. Among whites, the rate of HPV-related cancers is rising faster in males, which may reflect trends in sexual practices [16].
Dietary habits consistently demonstrate an effect on oral cavity and oropharyngeal cancer risk. Consumption of fruits and vegetables is protective, while red meats, dairy, and potatoes confer greater risk. Dietary intake may interact with tobacco and alcohol use in relation to cancer risk [17].
Individuals with a weakened immune system lose the ability to defend against infections and environmental toxins or eradicate abnormal host cells that have undergone malignant transformation. Within this population, transplant patients who remain on long-term immunosuppressive therapy are at the highest risk of developing malignancy, of which a small percentage develops in the head and neck. Immunosuppressive agents may also directly cause oncogenic threat. No specific therapy regimen has been shown to lower cancer risk [18]. Patients who undergo hematopoietic stem cell transplantation are at higher risk for OCSCC, which may be related to a high rate of graft-versus-host disease (GVHD) [19].
Some genetic syndromes predispose to cancer. Fanconi anemia, a rare inherited defect of DNA repair, is associated primarily with leukemias but may cause OCSCC. Xeroderma pigmentosum is another autosomal recessive defect of DNA repair, especially to damage from UV radiation. Patients are prone to developing cancers of the skin, lip, tongue, and buccal mucosa. Dyskeratosis congenita, also known as Zinsser-Cole-Engman syndrome, is a rare inherited disorder of telomere regulation. This disease clinically manifests as premature aging, oral leukoplakias, skin hyperpigmentation, nail dystrophy, and bone marrow failure. These patients are highly susceptible to many malignancies including those of the oral cavity.
3.2 Potentially Malignant Diseases, Formerly Known as “Precancerous Lesions,” of the Oral Cavity
Lesions with the ability to progress to malignancy are termed potentially malignant diseases (PMD). Previously known as “precancerous lesions,” this nomenclature is now discouraged as it implies inevitable transformation. In the oral cavity, the most common PMDs are leukoplakia and erythroplakia.
Leukoplakia presents as a white mucosal plaque in which cancer and other causes have been ruled out by a biopsy. Leukoplakia is found in 2 % of the worldwide population and is more common in smokers and tobacco chewers [20]. It is classified into homogenous and nonhomogenous types. Homogenous leukoplakia is often slightly raised and has a consistent texture. Nonhomogenous leukoplakia have an irregular texture and may be papular or nodular. Proliferative verrucous leukoplakia, a subtype of nonhomogenous leukoplakia, may be exophytic and carries a higher risk of malignancy. The overall risk of malignant transformation varies from 1 to 20 % and may occur decades after the diagnosis [21]. Erythroplakia is an erythematous red plaque not attributed to other causes. It most commonly presents as a flat or depressed lesion in the tongue, FOM, or buccal mucosa. There may be associated burning or pain. Alcohol and/or tobacco use is usually present in the history. Although less common than leukoplakia, it has a much higher risk of malignant transformation, between 35 and 67 % [22]. Lesions that are a mixture of red and white are termed erythroleukoplakia. The clinical behavior is similar to that of erythroplakia. Leukoplakias and erythroplakias are routinely removed surgically due to their malignant potential. Recurrence is common after excision, and lifelong follow-up is advocated by some.
GVHD patients are prone to develop premalignant oral mucosal lichenoid lesions, which are characterized by lacy white lines (Wickham’s striae) or persistent ulcerations, papules, plaques, or atrophic areas. The underlying etiology is a T-cell-mediated autoimmune response targeting epithelial cells. In the absence of a triggering factor, it is termed lichen planus. The risk of malignant transformation is estimated to be 1–3 % over 10 years and is not affected by tobacco or alcohol use, but may be higher with concomitant HPV infection [23].
3.3 Diagnosis
The superficial location of the oral cavity may facilitate early detection of malignant and premalignant lesions. Most patients are symptomatic on initial presentation. A common complaint is a persistent mass or ulcer that does not resolve despite antibiotic and/or steroid therapy and which may be associated with a painful or burning sensation. Other symptoms may be related to local effects of the tumor, including dysphagia due to impaired tongue mobility or obstruction of the oral cavity, neuropathies due to infiltration of the hypoglossal nerve or chorda tympani, loose dentition due to mandibular bone invasion, or hemoptysis due to a friable tumor surface. Infrequently, the patient complains only of a slow-growing mass in the neck, with the primary tumor site identified only after further evaluation. The history is usually suggestive of the diagnosis. Advanced age, history of heavy alcohol and/or tobacco use, HPV positivity, or other previously discussed risk factors should raise clinical suspicion for malignancy. Systemic manifestations include malaise and unintentional weight loss, which may be a primary effect of the malignancy or secondary to impaired oral intake.
Physical examination usually reveals a suspicious lesion in the oral cavity. Lesions often appear as a white or red mass that may be ulcerative or exophytic and may readily bleed with manipulation. The most common subsites are the oral tongue and FOM. The patient should be asked to elevate the tongue to allow adequate assessment of the FOM. The oral cavity and oropharynx should be thoroughly palpated to detect submucosal masses that feel firm. Examination of the FOM requires bimanual palpation of the submandibular glands. The state of the dentition and mandible should be noted in regard to surgical considerations and prognosis for oral feeding. A full cranial nerve examination is performed, with particular attention paid to lower cranial nerves. Tumor may spread to the hypoglossal nerve causing tongue weakness and deviation, the lingual nerve causing numbness of the oral tongue, the chorda tympani nerve causing dysgeusia (distorted sense of taste), or the glossopharyngeal nerve causing otalgia (ear pain). Biopsy of the lesion may be taken at this time to confirm the diagnosis.
Examination of the neck includes inspection and palpation for lymphadenopathy. Masses that are firm, immobile, non-tender, and persistent are highly concerning for malignancy. The most widely used classification system for the cervical lymph nodes was originally developed by the Memorial Sloan Kettering Cancer Center (Fig. 3.2) [24]. OCSCC most frequently involves the lymph node groups at Level I (and submandibular nodes), Level II (upper jugular chain and jugulodigastric nodes), and Level III (middle jugular chain nodes). Levels I, II, and V are further divided into subzones A and B, although further discussion falls outside of the scope of this chapter. Suspicious lymph nodes may be biopsied with or without ultrasound guidance. Fine-needle aspiration biopsy is performed using a 21–23-gauge needle to minimize risk of tumor seeding.
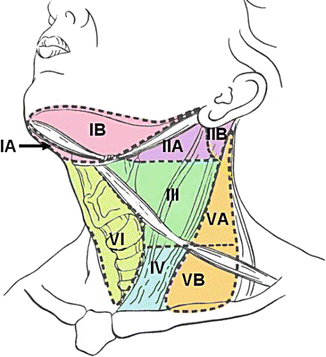

Fig. 3.2
Cervical lymph node groups (Reprinted in its original form from the Open Access Subset of PubMed Central, U.S. National Library of Medicine)
In-office examination of the upper aerodigestive tract is performed to assess tumor extension into deeper structures and to detect synchronous tumors. Traditionally, a mirror is placed into the oropharynx and angled inferiorly to indirectly examine the hypopharynx and larynx. More recently, flexible laryngoscopy has become the procedure of choice in many offices. This has the advantages of a magnified view and the ability to record images or video. After application of topical anesthesia, a flexible illuminated scope is inserted through the nose or mouth to visualize the oropharynx, hypopharynx, and larynx. The nasal cavity and nasopharynx may also be examined if inserted through the nose. Mucosal and submucosal lesions may be readily identified. Important findings to note include tumor size, gross extension to nearby structures, or vocal fold immobilization. Airway obstruction may result from mass effect of the primary tumor or an enlarged cervical lymph node and may mandate urgent surgical intervention
3.3.1 Staging
Accurate staging provides important prognostic information and guides treatment. The American Joint Committee on Cancer (AJCC) establishes guidelines for staging of oral cavity and lip cancers using the TNM classification based on characteristics of the primary tumor (T), regional nodal metastases (N), and distant metastases (M) (Table 3.1) [25].
Table 3.1
American Joint Committee on Cancer (AJCC) TNM classification of oral cavity cancer
|
Primary tumor (T)
|
|
|
TX
|
Cannot be assessed
|
|
T0
|
No evidence of primary tumor
|
|
Tis
|
Carcinoma in situ
|
|
T1
|
Tumor ≤2 cm in greatest dimension
|
|
T2
|
Tumor >2 cm but ≤4 cm in greatest dimension
|
|
T3
|
Tumor >4 cm in greatest dimension
|
|
T4a
|
Tumor invades adjacent structures only (e.g., through cortical bone [mandible or maxilla], into deep [extrinsic] muscle of tongue, maxillary sinus, or skin of face)
|
|
T4b
|
Tumor invades masticator space, pterygoid plates, or skull base and/or encases internal carotid artery
|
|
Regional lymph nodes (N)
|
|
|
NX
|
Cannot be assessed
|
|
N0
|
No regional lymph node metastasis
|
|
N1
|
Metastasis in a single ipsilateral lymph node, ≤3 cm in greatest dimension
|
|
N2
|
Metastasis in multiple lymph nodes, none >6 cm in greatest dimension
|
|
N2a
|
Metastasis in a single ipsilateral lymph node, >3 cm but ≤6 cm in greatest dimension
|
|
N2b
|
Metastasis in multiple ipsilateral lymph nodes, none >6 cm in greatest dimension
|
|
N2c
|
Metastasis in bilateral or contralateral lymph nodes, none >6 cm in greatest dimension
|
|
N3
|
Metastasis in a lymph node >6 cm in greatest dimension
|
|
Distant metastasis
|
|
|
MX
|
Unable to assess
|
|
M0
|
No distant metastasis
|
|
M1
|
Distant metastasis
|
Direct examination of the upper aerodigestive tract with the patient under general anesthesia permits a thorough assessment of tumor size and extension for primary tumor (T) staging. This procedure, known as triple endoscopy or panendoscopy, consists of direct laryngoscopy, bronchoscopy, and esophagoscopy. Any suspicious lesions may be biopsied at this time. A coexisting malignancy of the head and neck discovered within six months of the initial malignancy is termed a synchronous second primary tumor (SPT). Synchronous SPTs are detected in 1–7 % of patients [26, 27]. OCSCCs may be associated with higher rates of SPTs compared to other head and neck sites, most often in the esophagus or elsewhere within the oral cavity [28].
Radiological imaging supplements panendoscopy by assessing deep tissue involvement to provide information for tumor (T), lymph node (N), and distant metastasis (M) staging. Computed tomography (CT) and/or magnetic resonance imaging (MRI) studies from the skull base to the thoracic inlet are performed with intravenous contrast to provide detailed images of the oral cavity and neck. CT provides information on primary tumor extension, bone erosion, and regional nodal metastases. MRI may be preferable in patients who have dental implants, involvement of deep tongue musculature, or with suspected perineural spread. Generally, malignant tumors appear on imaging as a poorly circumscribed mass with irregular borders that enhance with contrast and may invade rather than push on adjacent structures.
Radiological signs of nodal metastasis include pathological enlargement, central necrosis, loss of normal ovoid shape, loss of a fatty hilum, and extracapsular spread. Ultrasonography rather than CT imaging of the cervical lymph nodes is more commonplace outside in North America. Imaging of the chest with plain films or CT is recommended to detect distant metastasis. Spread to the abdomen or pelvis is uncommon, and imaging is not routinely performed, although liver function tests should be obtained. Positive emission tomography-computed tomography (PET-CT) has a positive predictive value of up to 89 % and a false-positive rate of 8.3 % [29]. PET-CT is valuable for detecting unknown primary tumors and recurrent tumor, but may also be considered for patients with Stage III–IV cancer, of whom up to 30.9 % may have their treatment altered. The overall accuracy of preoperative staging is approximately 66–76 % for CT, MRI, and ultrasonography [30].
Early-stage malignancies are less than 4 cm in maximum dimension, do not invade key structures, and do not spread to lymph nodes or distant organs (Table 3.1). Determination of lymph node metastases is critical because this necessarily classifies the cancer as advanced stage. Locally advanced tumors classified as T4b are surgically unresectable due to intimate involvement of critical structures and/or difficulty of obtaining negative surgical margins. Tumors within the masticator space, pterygoid plates, skull base, nasopharynx, prevertebral fascia, or encasing the internal carotid artery are considered unresectable. On initial presentation, 31 % are diagnosed with localized disease, 47 % with regional metastasis, and 18 % with distant metastasis. The primary site remains unknown in 5 % after clinical examination and imaging [2].
3.3.2 National Comprehensive Cancer Network® Guidelines
The National Comprehensive Cancer Network® (NCCN) publishes clinical practice guidelines for the workup, staging, and treatment of head and neck malignancies by site [31]. Current guidelines for workup of oral cavity cancer include obtaining a thorough history, performing a complete head and neck examination with mirror or fiberoptic exam as indicated, and obtaining a tissue biopsy to confirm the diagnosis. On the initial visit, smoking cessation counseling should be provided as indicated and the patient should be screened for depression. Patients should be referred for dental evaluation and possible imaging. Other referrals to consider include nutrition, speech, and swallowing evaluation as indicated. Early initiation of swallow evaluation and therapy is encouraged to maximize postoperative swallow function and quality of life. Patients with head and neck cancer are often of advanced age and have multiple medical comorbidities. Preanesthesia evaluation may be necessary to identify perioperative risks and determine candidacy for surgery. The nutritional and health status of the patient should be optimized. The next steps in the workup for clinical staging are panendoscopy under anesthesia with directed biopsies and radiological imaging of the primary, neck, and chest as indicated. PET-CT is an option for those with Stage III–IV diseases and may upstage. Clinical staging is based on the AJCC TNM classification system (Table 3.1) [25].
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


